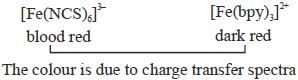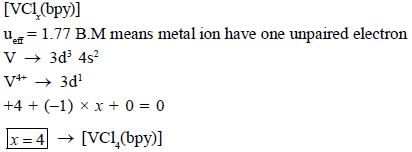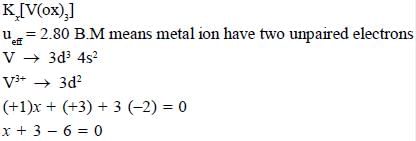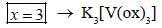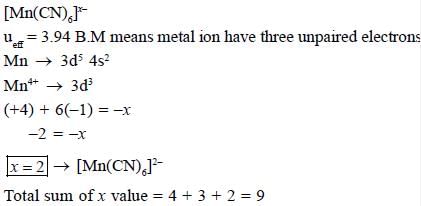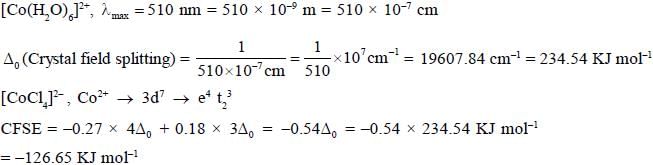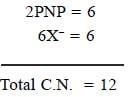Coordination Chemistry - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Coordination Chemistry
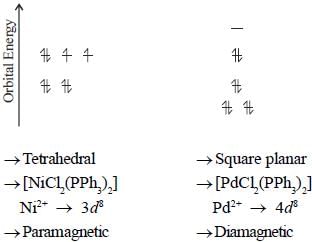
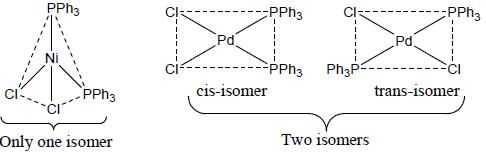
A complex of Ni(ll), [NiCl2(PPh3)2] is paramagnetic, the analogous complex of Palladium (II) is diamagnetic, the number of isomers shown by both complexes respectively are
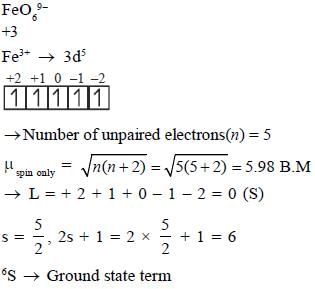
The spin-only magnetic moment and the spectroscopic ground state term symbol of iron centre in FeO69- ion respectively are
Among the following metal complexes in order of their increasing hydration energy.
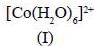
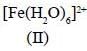
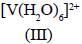
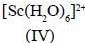
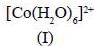
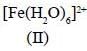
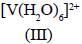
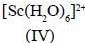
The true statement about base hydrolysis of [Co(CN)5JsBr]3- is
In square planar complexes which of the d -orbital / orbitals get direct interaction with ligand orbitals
The Δ0 value of [Co(NH3)6]3+ is 22,870 cm -1. The Δ0 value of [CoCH2O6]3+ and [C oF6]3- - compared to [Co(NH3)6]3+ respectively are
The total number of micro states for 3F, 3P and 1D is____________
The oxidation state of Ru metal ion in [Ru(Terpy)2)J (PF6)2 is__________
Potassium ferrocyanide reacts with nitric acid to form product A. Which further reacts with sodium carbonate to form product B. The total number of elections in t2g orbitals of metalion present in product B is
Among the following ligands, the number of ligands that can not act as ambidentate ligands is_________
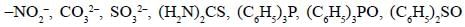
Among the following complexes, the correct trend of Pt-N bond strength is
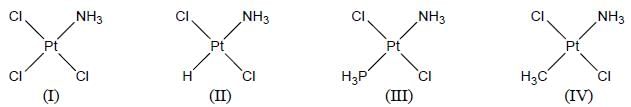
The correct trend of rate of electron transfer reaction between the complexes [CoC NH3),5X]2+/ [Co(H2O)6]2+ with variation of X is
The spmel structure of Cr2FeO4 has cubic close packed arrangement of oxide ions. The fractions of the tetrahedral and octahedral sites occupied by cation respectively are
Which of the following octahedral complex exhibits tetragonal compression
The colour and tlie mason for the deep coloration of [Fe(NCS)6]3- and [Fe(bpy)3]2+ respectively are
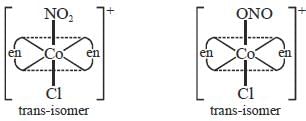
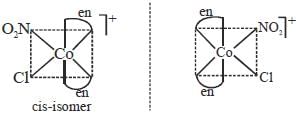
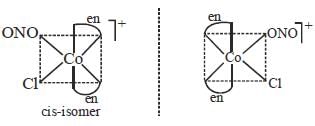
The total number of isomers possible for the molecule [Co(en)2Cl(NO2)]+ is___________
The total sum of x value of the following complexes is __________________
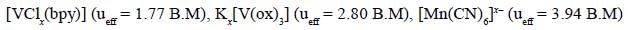
A complex [Co(h2O)6]2+ has an absorption maxima at 510 nm. The crystal field stabilisation energy for [CoC14]2- (in KJ mol-1) is _______________(Ignoring stabilisation charge)
In [Ni(PNP)X]2 [NiX4] complex, the total coordination number of metal ion is______________
(X is a uninegative monodentate ligand)
|
18 docs|37 tests
|



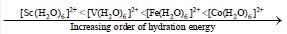
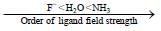
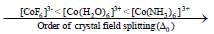

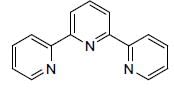
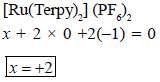

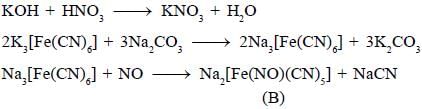
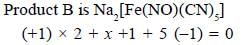
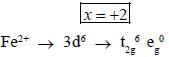
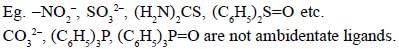
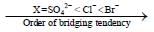
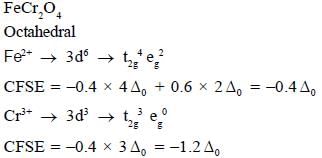
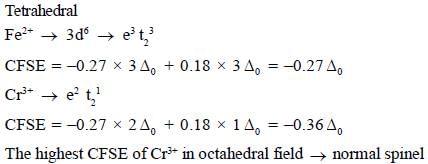
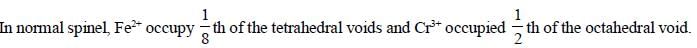
 which is responsible for blue colour.
which is responsible for blue colour.