Coordination Compounds (Chapter Test - Medical) - Class 12 MCQ
20 Questions MCQ Test - Coordination Compounds (Chapter Test - Medical)
Which one is the most likely structure of CrCI3.6H2O, if 1/3 of total chlorine of the compound is precipitated by adding AgNO3 to its aqueous solution ?
The coordination number and the oxidation state of element E in the complex [E(en)2(C2O4)]NO2
are,respectively
The oxidation state of central metal ion and magnetic moment of brown ring complex is
According to the postulates of Werner’s theory for coordination compounds
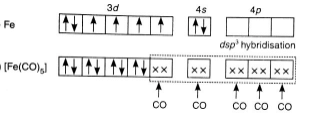
The chemical formula for iron(III)hexacyanoferrate(II) is
Which of the following is correctly matched?
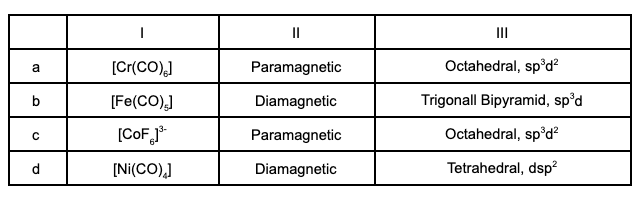
Which of the following is incorrectly matched ?
A complex cation is formed by Pt (in same oxidation state) with ligands (in proper number so that coordination number of Pt becomes six). Which of the following can be its correct IUPAC name ?
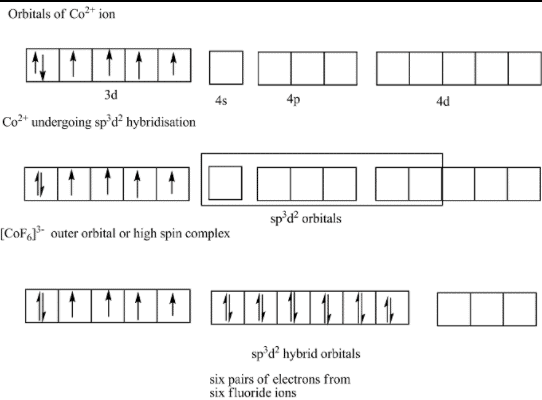
Which of the following has highest paramagnetic behaviour ?
Which order is correct in spectrochemical series of ligands?
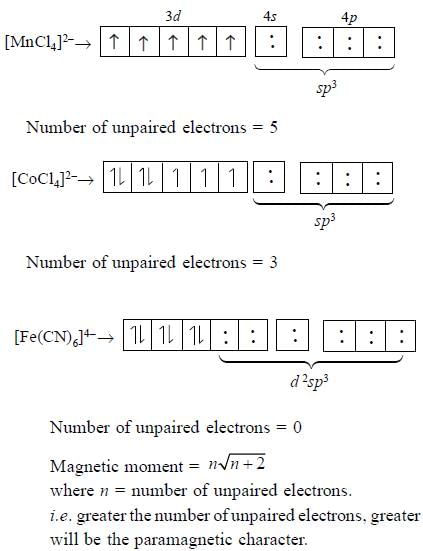
The correct order of magnetic moments (spin only values in B.M.) among is (Atomic no.s : Mn =25, Fe =
26,Co = 27)
When HgI2 is added to excess of aqueous KI, mercury largely exists as
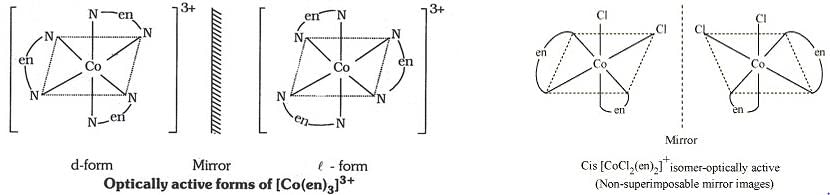
In which of the following pairs both the complexes show optical isomerism ?