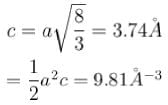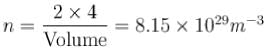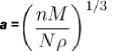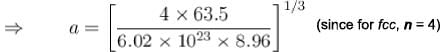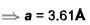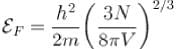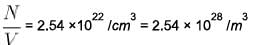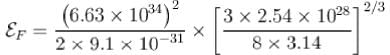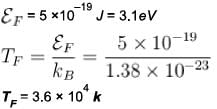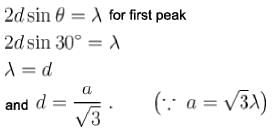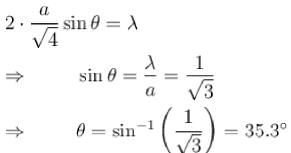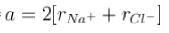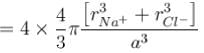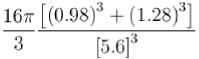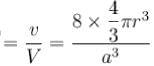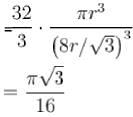Crystals NAT Level – 2 - Physics MCQ
10 Questions MCQ Test - Crystals NAT Level – 2
The probability that a state which is 0.2eV above the fermi energy in a metal atom at 700K is?
Be has hexagonal closed packed structure with smaller lattice parameter equal to 2.29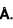 . Concentration of electron in Be crystal is in terms of 1029 m-3 .
. Concentration of electron in Be crystal is in terms of 1029 m-3 .
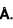 . Concentration of electron in Be crystal is in terms of 1029 m-3 .
. Concentration of electron in Be crystal is in terms of 1029 m-3 .A copper wire of uniform cross-sectional area 10 x 10-6 m-2 carries a current of 1 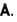 Assuming that each copper atom contributes one electron to the electron gas, the drift velocity of free electron in units of µm/sec is :
Assuming that each copper atom contributes one electron to the electron gas, the drift velocity of free electron in units of µm/sec is :
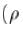 of copper is 8.94 x 103 kg/m3 and its atomic mass is 1.05 x 10-25 kg).
of copper is 8.94 x 103 kg/m3 and its atomic mass is 1.05 x 10-25 kg).
 Assuming that each copper atom contributes one electron to the electron gas, the drift velocity of free electron in units of µm/sec is :
Assuming that each copper atom contributes one electron to the electron gas, the drift velocity of free electron in units of µm/sec is :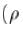 of copper is 8.94 x 103 kg/m3 and its atomic mass is 1.05 x 10-25 kg).
of copper is 8.94 x 103 kg/m3 and its atomic mass is 1.05 x 10-25 kg).Consider a crystal of fee structure. The density of the crystal is 8.96 glcm3 and atomic weight is 63.5. Then what is the lattice
constant for the crystal (in  )?
)?
The distance between the adjacent atomic planes in CaCO3 is 0.3 nm. The smallest angle of Bragg scattering for 0.03 nm X -ray is (in degrees)
Sodium contains 2.54 x1022 free electrons per cm3 ( given = 6.63 x 10 -34 J-s, m= 9 .11 x 10-31 kg , kB = 1.38 x 10-23 JIK and 1eV = 1.6 x 10-19 J ) , then Fermi D 4 temperature is given in terms of 104 K as?
In a powder diffraction pattern recorded from a face-centred cubic sample using X- rays. The first peak appears at 30°. The second peak will appear at what angle (degrees)
Masses of ionic radii of Na+ and CI– in NaCI crystals are 22.99 amu, 0.98A and 35.45 amu, 1.82A respectively. What is the packing fraction of NaCI?
Given that density of sodium is 0.971 g/cm3 and atomic weight is 23. If h = 6.63 * 10¯34 Js, mass of electron = 9.11 x 10-31 kg and Avogadro’s number is 6.02 x 1023 atoms/mol. Assuming each sodium atom contribute one electron to the electron gas. What is the electron density in units of 1028 Im3 ?
Packing fraction of diamond cubic crystal structure is ____ % ?


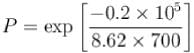
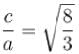 for hexagonal close packing
for hexagonal close packing