GATE -2018 Test-First (Chemistry) - IIT JAM MCQ
30 Questions MCQ Test - GATE -2018 Test-First (Chemistry)
In an irreversible process taking place at constant T and P and in which only pressure volume work is being done, the change in Gibbs free energy (dG) and change in entropy (dS) satisfy the criteria:
The un-normalized radial wave function of a certain hydrogen atom eigenstate is 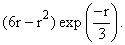
A possible angular part of the eigenstate is:
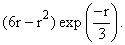
If the pre-exponential factor in Arrhenius equation is 1.6 × 1012s–1, the value of the rate constant at extremely high temperature will be close to:
1 mole of gas expanded isothermal and reversibly from 5L to 10 L. What is the enthalpy change.............
The distance between 3rd and 2nd orbit of hydrogen atom is:
The equilibrium 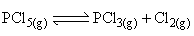 is attained at 25°C in a closed container and an inert gas He is introduced. Which of the following statements are correct:
is attained at 25°C in a closed container and an inert gas He is introduced. Which of the following statements are correct:
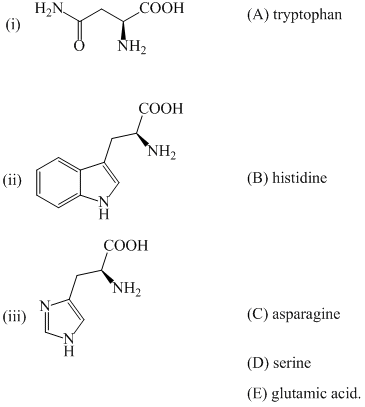
The number of framework electron pairs present in the borane cluster [B12H12]2–
Which of the following halides is least stable and has doubtful existence?
The rotational partition function of a diatomic molecule with energy levels corresponding to
J = 0 and 1 (where ε is a constant).
Yellow colour of Ce4+ and blood red colour of Sm2+ due to respectively:
Fraction of total volume occupied by atoms in a simple cubic cell is:
A plot of 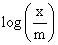 against log P for the adsorption of a gas on a solid gives a straight line with slope equal to:
against log P for the adsorption of a gas on a solid gives a straight line with slope equal to:
The lowest energy state of the (1s)2(2s)1(3s)1 configuration of Be is:
Which element have more observed magnetic moment than calculated:
Predict the inverse spinal structures, are among given below:
(I) NiFe2O4 (II) ZnFe2O4 (III) Mn3O4 (IV) CoFe2O4
Amongst the following, the group that is bound to the metal ion in coenzyme B12 is:
If Δ0 is the octahedral splitting energy and P is the electron pairing energy, then the crystal-field stabilization energy [CFSE) of [Co(NH3)6]2+ is:
How many 13C signal will observe in below given compound:

Arrange the following compounds in decreasing order of stretching frequency of C=O
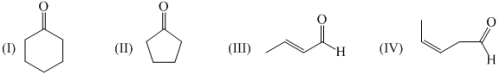
Choose the correct statements regarding given below compounds:
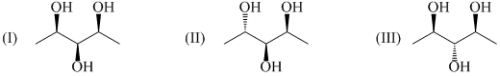
The major product formed in the following reaction is:
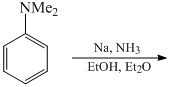
Arrange the following compound in decreasing order of reactivity of alkene for expoxidation reaction by peoxy acids:
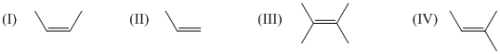
According to Wade's theory the anion [B12H12]2– adopts:
The degeneracy of an excited state of a particle in 3-dimensinal cubic box with energy three times its ground state energy is:
A buffer solution can be prepared from a mixture of:
(I) Sodium acetate and acetic acid in water
(II) Sodium acetate and HCl in water
(III) Ammonia and ammonium chloride in water.
(IV) Ammonia and sodium hydroxide in water
The resonance Raman stretching frequencies (in cm–1) of the bound O2 species in oxy-hemerythrin and oxy-hemoglobin, respectively, are:
M1 and M2 are the slopes 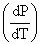 of the solid-liquid equilibrium lines in the p-T phase diagrams of H2O and CO2 respectively for P < 10 atm the value of M1 and M2 are:
of the solid-liquid equilibrium lines in the p-T phase diagrams of H2O and CO2 respectively for P < 10 atm the value of M1 and M2 are:



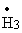 molecule become:
molecule become: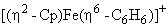 will give:
will give: