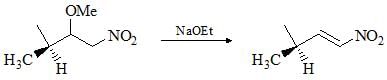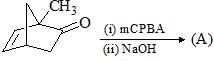GATE Chemistry Mock Test - 2 - GATE Chemistry MCQ
30 Questions MCQ Test GATE Chemistry Mock Test Series - GATE Chemistry Mock Test - 2
A student is required to demonstrate a high level of comprehension of the subject, especially in the social sciences.The word closest in meaning to comprehension is
Choose the most appropriate world from the options given below to complete the following sentence.One of this biggest_____________was his ability to forgive
Choose the most appropriate word from the options given below to complete the following sentenceThe principal presented the chief guest with a ______________as token of appreciation.
Choose the appropriate word/phrase, out of the four options given below, to complete the following sentence:Frogs______________________.
The overwhelming number of people infected with rabies in India has been flagged by the World Health Organization as a source of concern. It is estimated that inoculating 70% of pets and stray dogs against rabies can lead to a significant reduction in the number of people infected with rabies.Which of the following can be logically inferred from the above sentences?
A flat is shared by four first year undergraduate students. They agreed to allow the oldest of them to enjoy some extra space in the flat. Manu is two months older than Sravan, who is three months younger than Trideep. Pavan is one month older than Sravan. Who should occupy the extra space in the flat?
Find the area bounded by the lines 3x+2y = 14, 2x–3y = 5 in the first quadrant.
A straight line is fit to a data set (ln x, y). This line intercepts the abscissa at ln x = 0.1 and has a slope of –0.02. What is the value of y at x = 5 from the fit?
S, T, U, V, W, X, Y and Z are seated around a circular table. T is neighbours are Y and V. Z is seated third to the left of T and second to the right of S, U is neighbours are S and Y; and T and W are not seated opposite each other. Who is third to the left of V?
Among the following complexes, the ones that show(s) chirality are:
I = [Ru(bipyridyl)3]+, II = [Cr(EDTA)]-, III = trans[Cr(Cl)2(oxalate)2]3-, IV = cis[Cr(Cl)2(oxalate)2]3-
Consider the following statements and choose the correct one(s):
(I) Electron density in the XY plane in 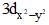 orbital is zero (II) Electron density in the XY plane in 3dz2 orbital is zero
orbital is zero (II) Electron density in the XY plane in 3dz2 orbital is zero
(III) 2s orbital has one nodal surface (IV) For 2pz orbital, YZ is the nodal plane,
Choose the correct product of the following reaction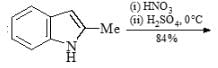
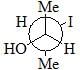
Which of the following is diastereomer of the given compound?
Which of the following is correct in reference to ferredoxin Fe2S2?
(I) In chloroplasts, Fe2S2 ferredoxins function as electron carriers in the photosynthetic electron transport chain
(II) They are proteins of around one hundred amino acids with four conserved cysteine residues to which the 2Fe-2S cluster is ligated
(III) The iron atoms are tetrahedrally coordinated both by inorganic sulfur atoms and by sulfurs of four conserved cysteine (Cys) residues.
Which of the following radial distribution graphs correspond to l = 2 for H atom for the least value of ‘n’ for which l = 2 is allowed?
The complex with the most intense color among following is?
The rotational partition function of a diatomic molecule with energy levels corresponding to J = 0 and 1 (where ε is a constant).
The d-bond is formed via the overlap of (considering z-axis as the inter-nuclear axis):
Noble gases (like He, Ne, Ar, Kr etc) are isolated from air. One of the steps is/are
18 electron rule is not followed in one of the following complexes?
Choose correct statement(s) about Actinides:
(I) All actinides are radioactive and release energy upon radioactive decay
(II) Actinides, especially those with a small number of 5f-electrons, are prone to hybridization.
(III) Like the lanthanides, all actinides are highly reactive with halogens and chalcogens; however, the actinides react more easily.
(IV) Chemically, actinium is similar to lanthanum, which is explained by their similar ionic radii and electronic structure.
Choose the correct staetement(s) in reference to Rotational spectroscopy:
Choose the major product formed in given chemical reaction sequence:
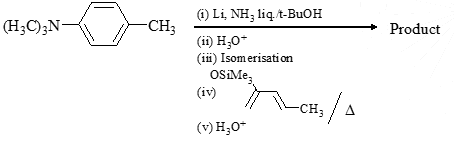
Choose the correct product of the following reaction:
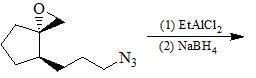
|
18 docs|37 tests
|