IIT JAM Biotechnology Past Year Papers - 2018 - IIT JAM MCQ
30 Questions MCQ Test - IIT JAM Biotechnology Past Year Papers - 2018
Which one of the following protozoan parasites belongs to the phylum Apicomplexa?
Which one of the following statements is CORRECT for Mycoplasma?
Which one of the following organelles is enclosed by a single membrane?
Pyramid of energy in a forest ecosystem is
In the feedback regulation of an enzyme, the end product binds to the
What is the source of electrons in photosynthesis?
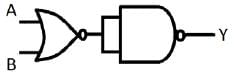
The logic operation (OR, AND, NOR or NAND) carried out by following circuit is
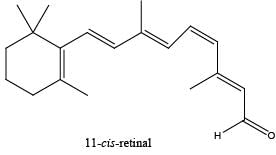
The reaction of 11-cis-retinal with the lysine residue of a specific protein forms the lightsensitive pigment in the cells of retina. The light-sensitive pigment is an
Viral capsids are made up of morphological subunits called capsomeres. One of the common capsomeres is icosahedral. The icosahedron is a regular polyhedron with
Which of the following feature(s) should be present in a protein to generate strong immune response (antibody production) in an animal?
I. At least one B-cell epitope
II. At least one T-cell epitope
III. Proteolytic cleavage site(s)
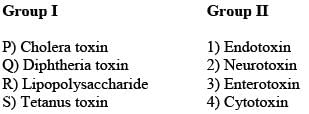
Match the entries in Group I with that in Group II.
Proenzyme pepsinogen is secreted from ‘P’ of gastric mucosa and converted into active enzyme pepsin on exposure to ‘Q’ secreted from ‘R’. Choose the CORRECT combination of P, Q and R.
When bacteria are grown in glucose-depleted media containing high concentration of lactose, expression of lac operon genes is activated by
Match the hormones in Group I with their functions in Group II

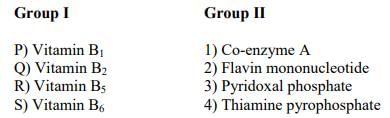
Match the entries in Group I with that in Group II

Match the entries in Group I with that in Group II
The number of three letter words, with or without meaning, which can be formed using letters of the word ‘VIRUS’ without repetition of letters is
What is the solution of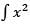 In xdx?
In xdx?
Given C is an arbitrary constant.
The area of an equilateral triangle with sides of length α is
Nucleus of a radioactive material can undergo beta decay with half life of 4 minutes. Suppose beta decay starts with 4096 nuclei at t = 0, the number of nuclei left after 20 minutes would be
Which one of the following shows the CORRECT relationship among velocity of light in a medium (v), permittivity of medium(ε)and magnetic permeability of medium(μ)?
A 30 µF capacitor is connected to a 240 V, 50 Hz source. If the frequency of the source is changed from 50 Hz to 200 Hz, the capacitive reactance of the capacitor will
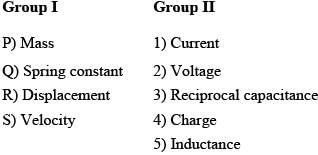
Match the entries in Group I (Mechanical system) with analogous quantities in Group II (Electrical system)
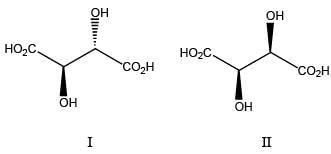
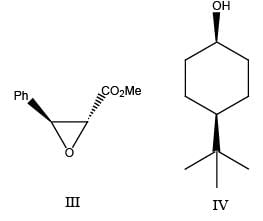
The achiral molecules among the following (I, II, III and IV) are
Match the entries in Group I with those in Group II

Which one of the following statements is CORRECT?
In the 1H NMR spectrum, which one of the following compounds will show a triplet?