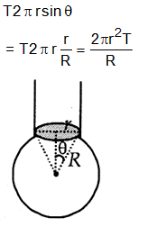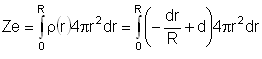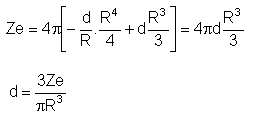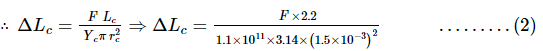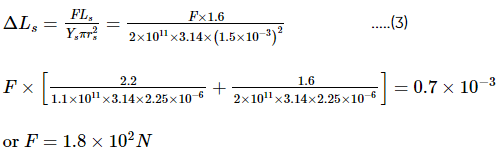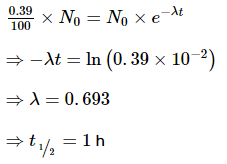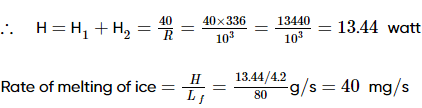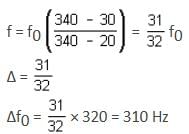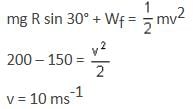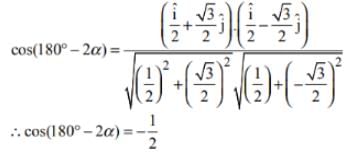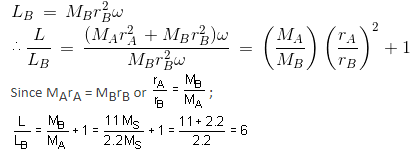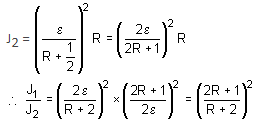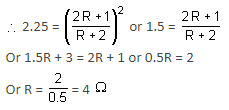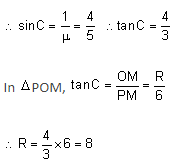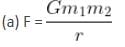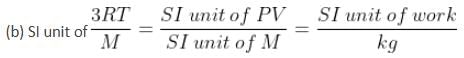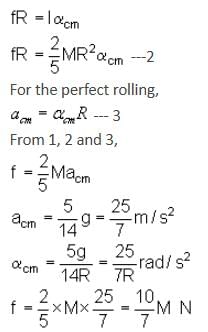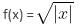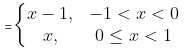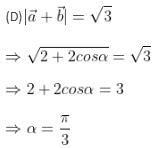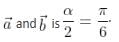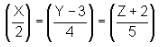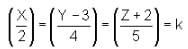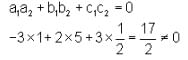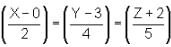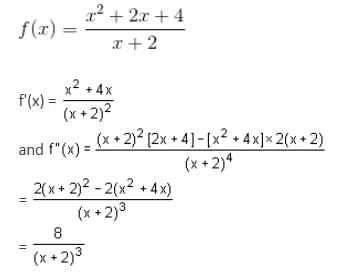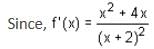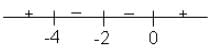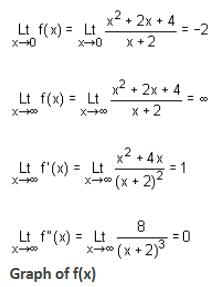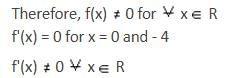JEE Advanced Mock Test - 4 (Paper I) - JEE MCQ
30 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Advanced Mock Test - 4 (Paper I)
When liquid medicine of density p is to be put in the eye it is done with the help of a dropper. As the bulb on the top of the dropper is pressed, a drop forms at the opening of the dropper. We wish to estimate the size of the drop. We first assume that the drop formed at the opening is spherical because that requires a minimum increase in its surface energy. To determine the size, we calculate the net vertical force due to the surface tension T when the radius of the drop is R. When this force becomes smaller than the weight of the drop, the drop gets detached from the dropper.
If the radius of the opening of the dropper is r, the vertical force due to the surface tension on the drop of radius R (assuming r << R)
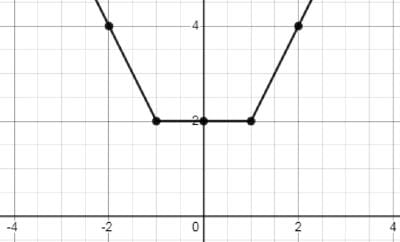
The nuclear charge (Ze) is non-uniformly distributed within a nucleus of radius R. The charge density p(r) [charge per unit volume] is dependent only on the radial distance r from the centre of the nucleus as shown in figure. The electric field is only along the radial direction.
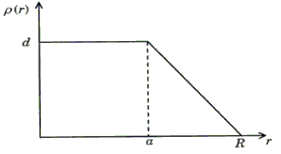
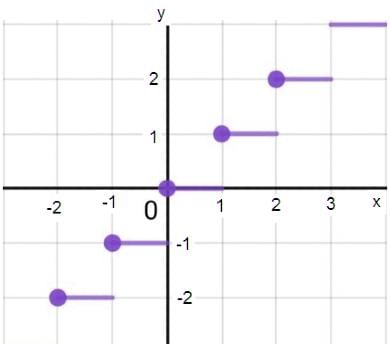
For a = 0, the value of d (maximum value of p as shown in the figure) is

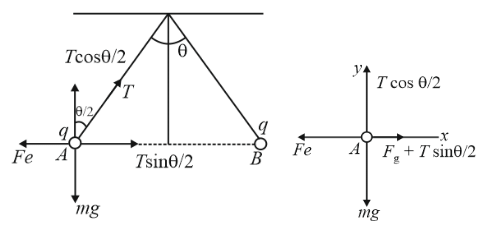
Two small equally charged spheres, each of mass m, are suspended from the same point by silk threads of length l. The distance between the spheres x << l. Find the rate dq/dt with which the charge leaks off each sphere if their approach velocity varies as v = a/√x , where a is a constant.
A composite wire of uniform diameter 3.0 mm consists of a copper wire of length 2.2 m and a steel wire of length 1.6 m which is stretched under a load by 0.7 mm. Calculate the load, given that the Young's modulus of elasticity for copper is 1.1 × 1011 N m–2 and that for steel is 2 × 1011 N m–2.
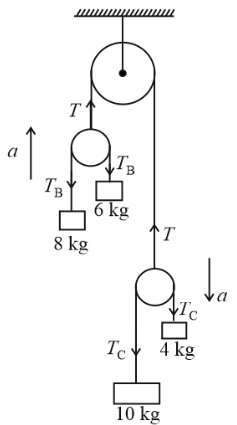
Consider the situation shown in figure and select the correct statement from the following.
It is observed that only 0.39% of the original radioactive sample remains un decayed after eight hours. Hence
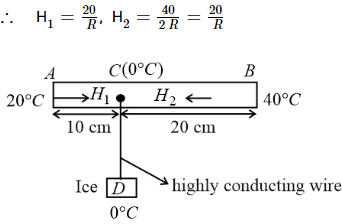
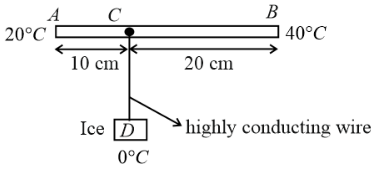
In the figure shown AB is rod of length 30 cm and area of cross-section 1 cm2 and thermal conductivity 336 S.I. units. The ends A and B are maintained at temperature 20°C and 40°C respectively. A point C of this rod is connected to a box D, containing ice at 0°C, through a highly conducting wire of negligible heat capacity. Find the rate at which ice melts in the box. [Assume latent heat of fusion for ice Lice = 80 cal g−1]
Two trains A and B are moving with speeds 20 m/s and 30 m/s respectively in the same direction on the same straight track, with B ahead of A. The engines are at the front ends. The engine of train A blows a long whistle.
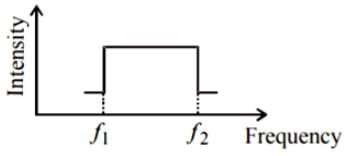
Assume that the sound of the whistle is composed of components varying in frequency from f1 = 800 Hz to f2 = 1,120 Hz, as shown in the figure. The spread in the frequency (highest frequency - lowest frequency) is thus 320 Hz. The speed of sound in still air is 340 m/s.
The spread of frequency (in Hz) as observed by the passengers in train B is
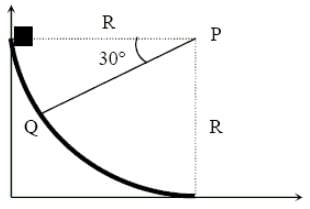
A small block of mass 1 kg is released from rest at the top of a rough track. The track is circular arc of radius 40 m. The block slides along the track without toppling and a frictional force acts on it in the direction opposite to the instantaneous velocity. The work done in overcoming the friction up to the point Q, as shown in the figure below, is 150 J. (Take the acceleration due to gravity, g = 10 m/s-2)
The speed (in ms-1) of the block when it reaches the point Q is
A ray of light travelling in the direction 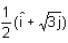 is incident on a plane mirror. After reflection, it travels along the direction
is incident on a plane mirror. After reflection, it travels along the direction 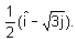 What is the angle of incidence (in degrees)?
What is the angle of incidence (in degrees)?
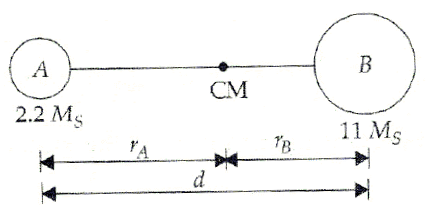
A binary star consists of two stars A (mass 2.2Ms) and B (mass 11MS), where Ms is the mass of the Sun. They are separated by distance d and are rotating about their centre of mass, which is stationary. The ratio of the total angular momentum of the binary star to the angular momentum of star B about the centre of mass is
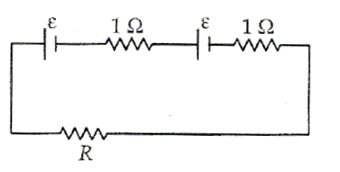
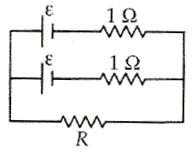
When two identical batteries of internal resistance 1 Ω each are connected in series across a resistor R, the rate of heat produced in R is J1. When the same batteries are connected in parallel across R, the rate is J2. If J1 = 2.25J,, then the value of R (in Ω) is
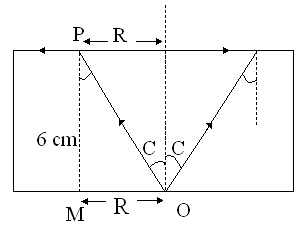
A large glass slab (μ = 5/4) of thickness 6 cm is placed over a point source of light on a plane surface. There is a bright circular patch of light on the top surface of the slab with radius R cm. What is the value of R?
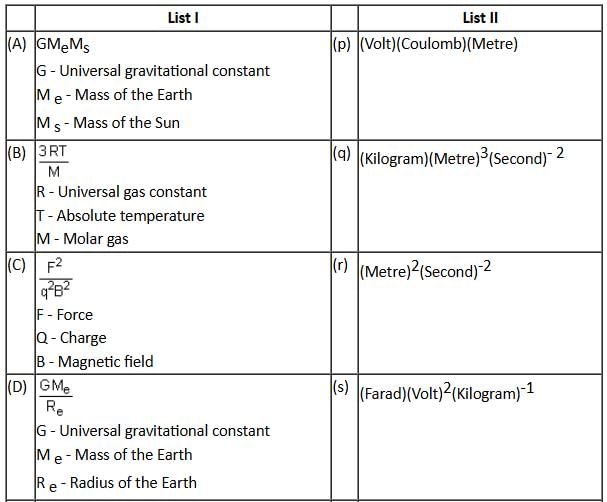
Answer the following by appropriately matching the lists based on the information given:
List I includes physical quantities and List II includes units for the physical quantities given in List I.
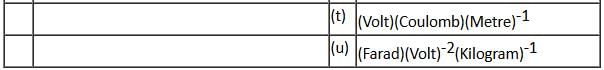
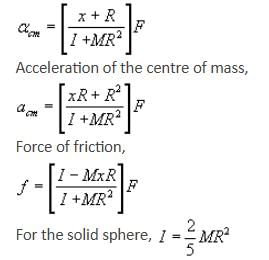
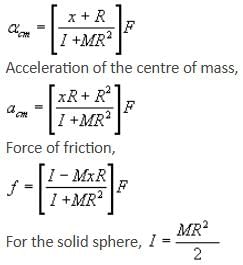
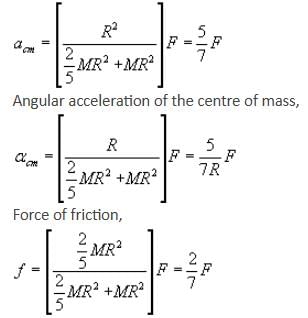
If the given rolling body is a solid sphere and force F is acting at a distance of R/2 above the centre, calculate acm, αcm and friction.
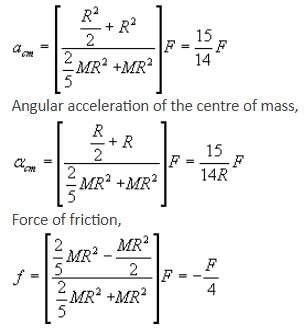
If the given rolling body is a ring and force F is passing through the centre of mass, calculate acm, αcm and friction.
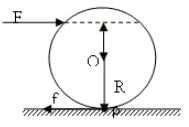
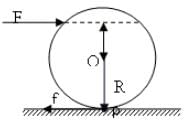
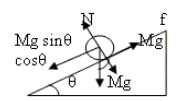
A solid sphere having mass 'M' and radius 'R' is placed on a rough inclined plane having inclination 30°. It moves down rolling without slipping. What will be the values of acm, αcm and friction?
Directions: The following question is based on the paragraph given below.
The noble gases have closed-shell electronic configuration and are monatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions.
The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2, +4 and +6. XeF4 reacts violently with water to give XeO3. The compound can also be prepared using XeF6 as the starting compound. The compounds of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell.
The chemical nature of the compounds XeF4 and XeF6 is expected to be
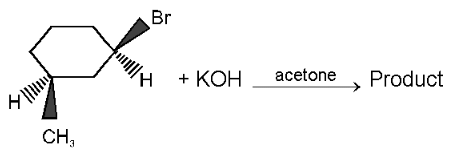
(1R, 3S)-Cis-1-Bromo-3-methyl cyclohexane. The product formed in the reaction is
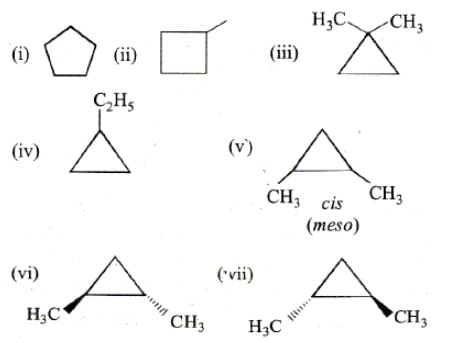
The total number of cyclic structural as well as stereoisomer possible for a compound with the molecular formula C5H10 is
Consider a reaction: aG + bH → products. When concentration of both the reactants G and H is doubled, the rate increases by eight times. However, when concentration of G is doubled keeping the concentration of H fixed, the rate is doubled. The overall order of the reaction is
The sum of the number of lone pairs of electrons on each central atom in the following species is
[TeBr6]2–, [BrF2]+, SNF3, and [XeF3]–
(Atomic numbers: N = 7, F = 9, S = 16, Br = 35, Te = 52, Xe = 54)
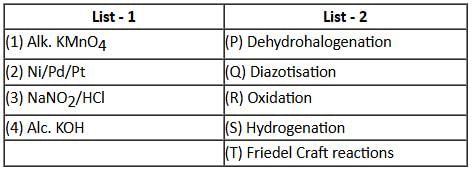
Match the reagents in List - 1 with their properties in List - 2, and choose the correct option.
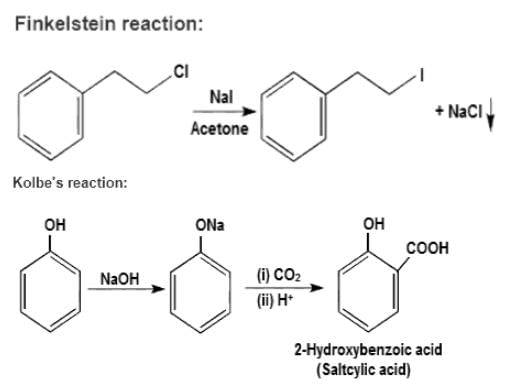
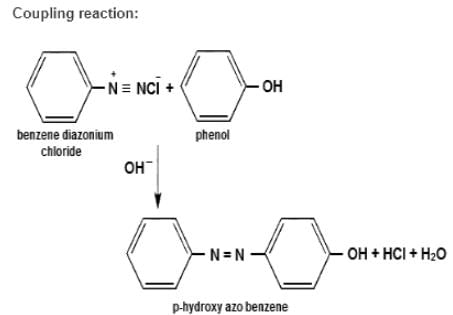
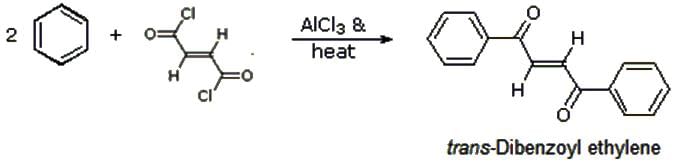
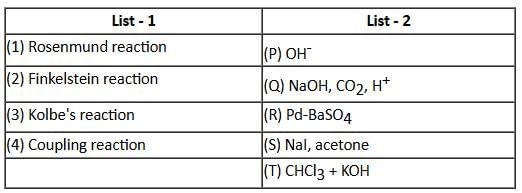
Match the organic name reactions in List - 1 with appropriate reagents for the reactions in List - 2, and choose the correct option.
Answer by appropriately matching the information given in the three columns of the following table:
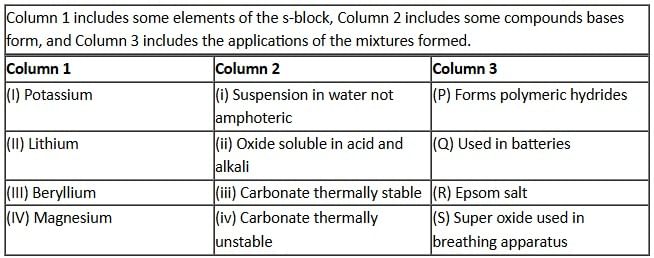
From the three columns, select the correct combination for the metal that is the weakest reducing agent.
Answer by appropriately matching the information given in the three columns of the following table:
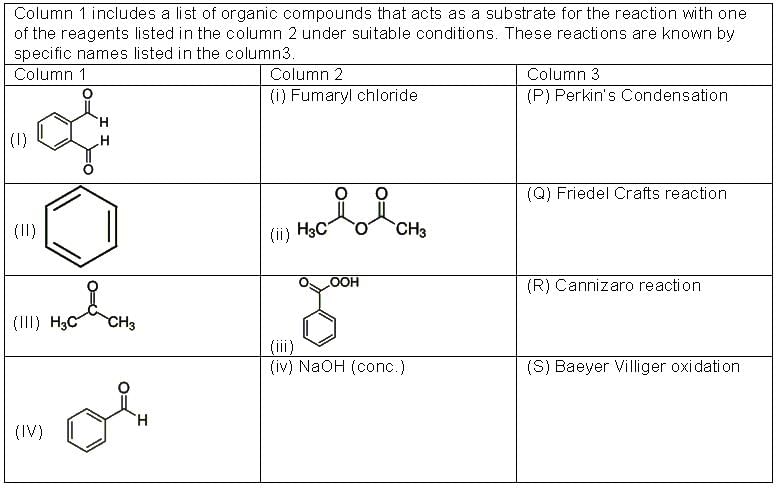
From the three columns, select the correct combination for the formation of a Diphenyl ketone.
In the following question, [x] denotes the greatest integer less than or equal to x.
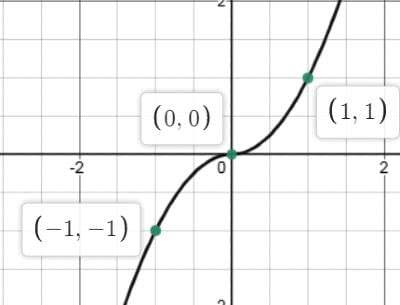
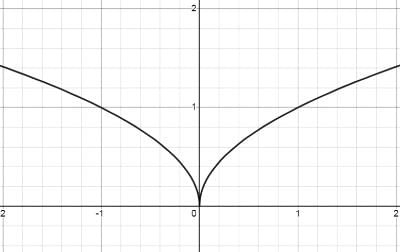
Which of the following options correctly matches the functions in Column I with their properties listed in Column II?
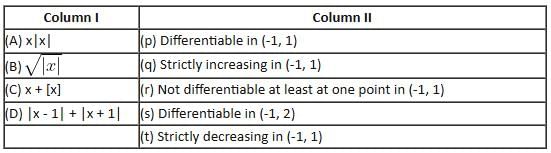
Match the statements/expressions given in Column I with the values given in Column II.
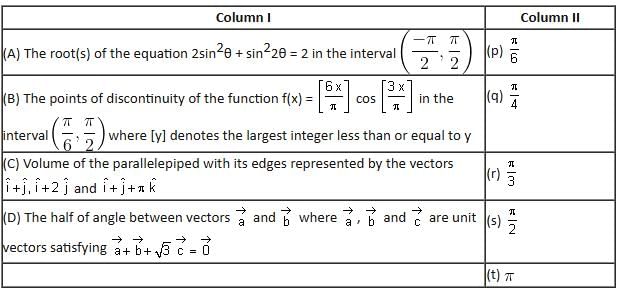
Which of the following is the only correct option?
Directions: Answer the following question by appropriately matching the information given in the three columns in the following table.
Here,
Column I contains equation of line
Column II contains any points in plane
Column III contains coordinates of foot of perpendicular when drawn from the points in column II
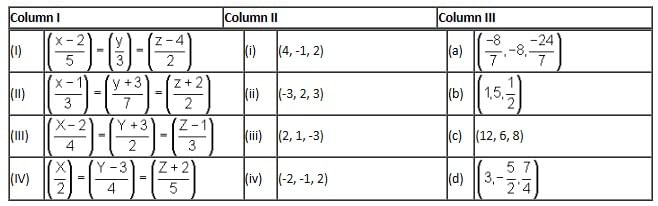
Which of the following combinations is incorrect?
Directions: Answer by appropriately matching the information given in the three columns of the following table.
Let
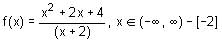
* Column 1 contains information about functional value of f(x), f'(x) and f ''(x).
* Column 2 contains information about limiting behaviour of f(x), f'(x) and f ''(x) at different points.
* Column 3 contains information about increasing/decreasing nature of f(x) and f'(x).
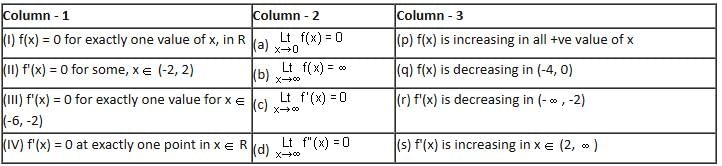
Which of the following option is the only incorrect combination?
|
356 docs|142 tests
|