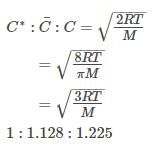JEE Main 2013 Question Paper with Solutions - JEE MCQ
30 Questions MCQ Test - JEE Main 2013 Question Paper with Solutions
PART A
Q. No. 1 -30 carry 4 marks each and 1 mark is deducted for every wrong answer.
Q. An unknown alcohol is treated with the “Lucas reagent” to determine whether the alcohol is primary,
secondary or tertiary. Which alcohol reacts fastest and by what mechanism:
secondary or tertiary. Which alcohol reacts fastest and by what mechanism:
The first ionization potential of Na is 5.1 eV. The value of electron gain enthalpy of Na+ will be:
Stability of the species 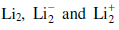 increases in the order of:
increases in the order of:
The molarity of a solution obtained by mixing 750 mL of 0.5 (M) HCl with 250 mL of 2(M)HCl will be:
Which of the following is the wrong statement?
Four successive members of the first row transition elements are listed below with atomic numbers. Which
one of them is expected to have the highest value?
A solution of (–) –1 – chloro –1 – phenylethane is toluene racemises slowly in the presence of a small
amount of SbCl5, due to the formation of :
The coagulating power of electrolytes having ions Na+, Al3+ and Ba2+ for arsenic sulphide sol increases in
the order:
How many litres of water must be added to 1 litre of an aqueous solution of HCl with a pH of 1 to create an
aqueous solution with pH of 2?
Which one of the following molecules is expected to exhibit diamagnetic behaviour?
Which of the following arrangements does not represent the correct order of the property stated against it?
Experimentally it was found that a metal oxide has formula M0.98O. Metal M, is present as M2+ and M3+ in
its oxide. Fraction of the metal which exists as M3+ would be:
compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass
390. The number of amino groups present per molecule of the former compound is:
Given
Based on the data given above, strongest oxidising agent will be:
Arrange the following compounds in order of decreasing acidity:
The rate of a reaction doubles when its temperature changes from 300K to 310K. Activation energy of such
a reaction will be:
(R = 8.314 JK–1 mol–1 and log 2 = 0.301)
Synthesis of each molecule of glucose in photosynthesis involves:
Which of the following complex species is not expected to exhibit optical isomerism?
A piston filled with 0.04 mol of an ideal gas expands reversibly from 50.0 mL to 375 mL at a constant
temperature of 37.00C. As it does so, it absorbs 208J of heat. The values of q and w for the process will be:
(R = 8.314 J/mol K) ( l n 7.5 = 2.01)
A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g of CO2. The empirical formula of
the hydrocarbon is:
The order of stability of the following carbocations:
is
Which of the following represents the correct order of increasing first ionization enthalpy for Ca, Ba, S, Se
and Ar?
For gaseous state, if most probable speed is denoated by C*, average speed by and mean square speed by C, then for a large number of molecules the ratios of these speeds are:
The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was:
Consider the following reaction:
The values of x, y and z in the reaction are, respectively:
Which of the following exists as covalent crystals in the solid state?
Compound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A)
gives a acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
Energy of an electron is given by Wavelength of light required to excite an
electron in an hydrogen atom from level n = 1 to n = 2 will be
(h = 6.62 × 10−34 Js and c = 3.0 × 108 ms−1)
An organic compound A upon reacting with NH3 gives B. On heating B gives C. C in presence of KOH
reacts with Br2 to give CH3CH2NH2. A is
In which of the following pairs of molecules/ions, both the species are not likely to exist?