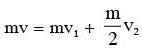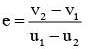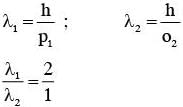JEE Main 2017 Question Paper with Solutions - JEE MCQ
30 Questions MCQ Test - JEE Main 2017 Question Paper with Solutions
A particle is executing simple harmonic motion with a time period T. AT time t = 0, it is at its position of equilibrium. The kinetic energy-time graph of the particle will look like
The temperature of an open room of volume 30 m3 increases from 17°C to 27°C due to sunshine. The atmospheric pressure in the room remains 1 × 105 Pa. If ni and nf are the number of molecules in the room before and after heating, then nf – ni will be :-
Which of the following statements is false ?
The following observations were taken for determining surface tensiton T of water by capillary method : Diameter of capilary, D = 1.25 × 10–2 m rise of water, h = 1.45 × 10–2 m
Using g = 9.80 m/s2 and the simplified relation N/m, the possible error in surface tension is closest to :
In amplitude modulation , sinusoidal carrier frequency used is denoted by ωc and the signal frequency is denoted by ωm. The bandwidth (Δωm) of the signal is such that Δωm << ωc. Which of the following frequencies is not contained in the modulated wave ?
A diverging lens with magnitude of focal length 25 cm is placed at a distance of 15 cm from a converging lens of magnitude of focal length 20 cm. A beam of parallel light falls on the diverging lens. The final image formed is :
The moment of inertia of a uniform cylinder of length l and radius R about its perpendicular bisector is I. What is the ratio l/R such that the moment of inertia is minimum ?
An electron beam is accelerated by a potential difference V to hit a metallic target to produce X-rays. It produces continuous as well as characteristic X - rays. If λmin is the smallest possible wavelength of X-ray in the spectrum, the variation of log λmin with log V is correctly represented in :
A radioactive nucleus A with a half life T, decays into a nucleus B. At t = 0, there is no nucleus B. At sometime t, the ratio of the number of B to that of A is 0.3. Then, t is given by :
An electric dipole has a fixed dipole moment which makes angle q with respect to x-axis. When subjected to an electric field
it experiences a torque
When subjected to another electric field
it experiences torque
. The angle q is :
In a common emitter amplifier circuit using an n-p-n transistor, the phase difference between the input and the output voltages will be :
Cp and Cv are specific heats at constant pressure and constant volume respectively. It is observed that Cp – Cv = a for hydrogen gas Cp – Cv = b for nitrogen gas The correct relation between a and b is :
A copper ball of mass 100 gm is at a temperature T. It is dropped in a copper calorimeter of mass 100 gm, filled with 170 gm of water at room temperature. Subsequently, the temperature of the system is found to be 75°C. T is given by : (Given : room temperature = 30° C, specific heat of copper = 0.1 cal/gm°C
A body of mass m = 10–2 kg is moving in a medium and experiences a frictional force F = –kv2. Its intial speed is v0 = 10 ms–1. If, after 10 s, its energy is 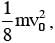 the value of k will be:-
the value of k will be:-
When a current of 5 mA is passed through a galvanometer having a coil of resistance 15 Ω, it shows full scale deflection. The value of the resistance to be put in series with the galvanometer to convert it into to voltmeter of range 0 -10 V is:
A slender uniform rod of mass M and length ℓ is pivoted at one end so that it can rotate in a vertical plane (see figure). There is negligible friction at the pivot. The free end is held vertically above the pivot and then released. The angular acceleration of the rod when it makes an angle θ with the vertical is :
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths r = λ1/λ2, is given by :
A man grows into a giant such that his linear dimensions increase by a factor of 9. Assuming that his density remains same, the stress in the leg will change by a factor of :
In a coil of resistance 100 Ω, a current is induced by changing the magnetic flux through it as shown in the figure. The magnitude of change in flux through the coil is :
In a Young's double slit experiment, slits are separated by 0.5 mm, and the screen is placed 150 cm away. A beam of light consisting of two wavelengths, 650 nm and 520 nm, is used to obtain interference fringes on the screen. The least distance from the common central maximum to the point where the bright fringes due to both the wavelengths coincide is :
A magnetic needle of magnetic moment 6.7 × 1 0 –2 Am2 and moment of inertia 7.5 × 10–6 kg m2 is performing simple harmonic oscillations in a magnetic field of 0.01 T. Time taken for 10 complete oscillations is :
The variation of acceleration due to gravity g with distance d from centre of the earth is best represented by (R = Earth's radius):
In the above circuit the current in each resistance is :
A particle A of mass m and initial velocity v collides with a particle B of mass (m/2) which is at rest. The collision is head on, and elastic. The ratio of the de–Broglie wavelengths λA to λB after the collision is :
An external pressure P is applied on a cube at 0°C so that it is equally compressed from all sides. K is the bulk modulus of the material of the cube and a is its coefficient of linear expansion. Suppose we want to bring the cube to its original size by heating. The temperature should be raised by
A time dependent force F = 6t acts on a particle of mass 1 kg. If the particle starts from rest, the work done by the force during the first 1 sec. will be :
An observer is moving with half the speed of light towards a stationary microwave source emitting waves at frequency 10 GHz. What is the frequency of the microwave measured by the observer? (speed of light = 3 × 108 ms–1)
In the given circuit diagram when the current reaches steady state in the circuit, the charge on the capacitor of capacitance C will be :
A capacitance of 2 μF is required in an electrical circuit across a potential difference of 1.0 kV. A large number of 1 μF capacitors are available which can withstand a potential difference of not more than 300 V. The minimum number of capacitors required to achieve this is :
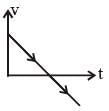
A body is t hrown vertically upwards. Which one of the following graphs correctly represent the velocity vs time?


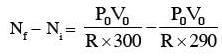
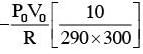
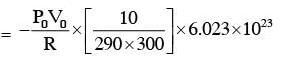

 .... (2)
.... (2)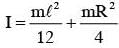
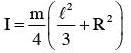 .....(1)]
.....(1)]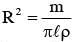 Put inequation (1)
Put inequation (1)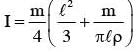
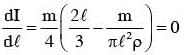
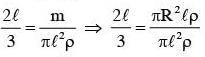
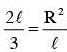
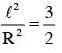
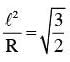
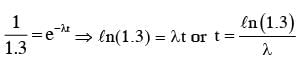
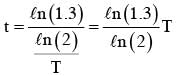
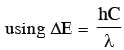
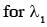
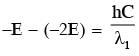
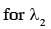
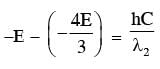
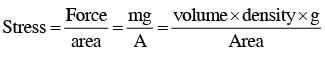
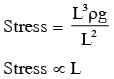
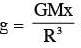 inside the Earth (straight line)
inside the Earth (straight line)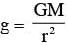 out side the Earth
out side the Earth