JEE Main Chemistry Mock Test- 7 - JEE MCQ
25 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Main Chemistry Mock Test- 7
Primary and secondary alcohols on action of red hot copper give
Which of the following gives blood red colour with KCNS ?
Given that
(i) C + O₂ → CO₂ ; ∆Ho = − xkJ,
(ii) 2CO + O₂ → 2CO₂ ; ∆Ho = −ykJ .
The enthalpy of formation of carbon monoxide will be
In the electrochemical cell Pt, H2 (g) 1 atm ∕ H+(I M) ∕ Cu2+ (I M) ∕ Cu(s) . Which one of the following statements is true?
pH of the solution at 250C is 2. If the pH is to be doubled then the hydronium ion concentration of the solution should be
Given that Ka for acetic acid as 1.8x10⁻5 and Kb of NH₄OH as 1.8x10⁻5 at 25oC,predict the nature of aqueous solution of ammonium acetate
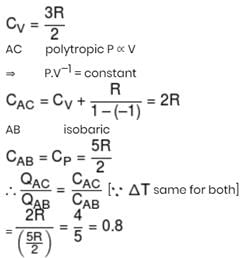
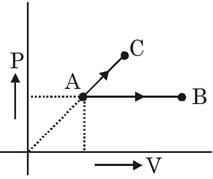
One mole ideal monoatomic gas is heated in two different processes according to path AB and AC. If temperature of state B and state C are equal. Calculate qAC/qAB
What is the molecular weight of the final product in the following reaction sequence
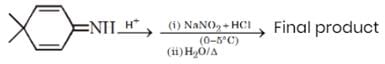
An ionic compound (A+B–) crystallizes in rock salt structure. If the ionic radii of A+ and B– is 200 pm and 400 pm respectively, then calculate distance between nearest anions in Å.
6.84 gm Al2(SO4)3 is needed to coagulate 2.5 L of As2S3 sol completely in 2.0 hrs. The coagulation value of Al2(SO4)3 in terms of millimoles per litre is :
[Atomic mass : Al = 27, S = 32].
The sum of Number of unpaired electrons in
[COCl6]–3, [Cr(NH3)6]+3 , [Zn(NH3)4]+2 →
|
356 docs|142 tests
|