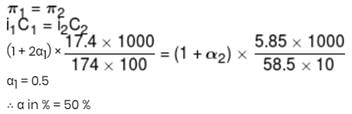JEE Main Chemistry Test- 4 - JEE MCQ
25 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Main Chemistry Test- 4
An organic compound ‘X’ on treatment with acidified 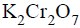 gives a compound ‘Y’ which reacts with
gives a compound ‘Y’ which reacts with 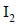 and sodium carbonate to form tri-iodomethane. The compound ‘X’ is
and sodium carbonate to form tri-iodomethane. The compound ‘X’ is
The reaction of with acetone and hydrolysis of the resulting product gives
17.4 % (mass / volume) K2SO4 solution at 27°C is isotonic to 5.85% (mass / volume) NaCl solution at 27°C. If NaCl is 100% ionised, what is % ionisation of K2SO4 in aq. solution?
Among the following ore how many example of carbonate ore:
lime stone, malachite, dolomite, Horn silver, Flourspar, Calamine, Cuprite.
Identify the element for given which have the most negative electron gain enthalpy. Write atomic number of that element as answer :-
P , S, Cl, F, Br
Element 'X' has maximum electron affinity in periodic table then find the number of elements which have low first ionisation energy as compared to element 'X' ?
F, Na, S, Si, P, Ar
If number of low spin complexes are 'X' then find the value of 1/x.
[Co(NH3)6]+3, [CoF6]–3, [Fe(CN)6]–4, [Fe(H2O)6]+2, [Cr(gly)3]0, [CoBr2Cl2]–2
|
356 docs|142 tests
|