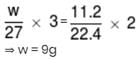JEE Main Chemistry Test- 7 - JEE MCQ
25 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Main Chemistry Test- 7
To which class of compounds do enzymes usually belong ?
XeF4 is prepared by heating Xe and F2 at 400oC in the ratio of
Which of the following polymer type is not classified on the basis of its application and properties?
On passing electric current through moten AlCl3, 11.2 litre of Cl2 is liberated at N.T.P. at anode. The quantity of aluminium deposited at cathode in g will be :- (At. wt. of Al = 27)
How many of the following are condensation copolymers ?
Nylon-6, nylon-66, Dacron, glyptal, buna-S, ABS, neoprene, PHBV, perlon-U.
A 250.0 ml sample of a 0.20 M Cr3+ is electrolyzed with a current of 96.5 A , so as to deposit Cr. If the remaining [Cr3+] is 0.1M, the time taken (in sec.) in the process.
How many statements are correct in the following -
(a) Reduction potential of Pb2+ is more than Zn2+
(b) Mercury cell is an example of primary cell
(c) Hydrogenation of ethene (C2H4) is a first order reaction
(d) order of wavelength γ-rays < X-ray < IR
(e) Molal elevation constant (Kb) of ethanol is more than carbon tetrachloride (CCl4)
(f) Sols of starch, gum, gelatin are postively charged.
(g) In coagulation of positive sol, the flocculating power is in order
: [Fe(CN)6]–4 > PO4–3 > SO4–2 > Cl–
(h) In Rhombohedral unit cell : a = b = c
Find out the ratio of incorrect and correct statements about H2O2 among the following?
1. In the pure state H2O2 is almost colorless (very pale blue)
2. Hydrogen peroxide has non planar structure in both gas phase & solid phase
3. 2-ethylanthraquinol react with water to give H2O2
4. H2O2 is used in pollution control
5. Dihedral angle of H2O2 is larger in gas phase compared to that in solid phase
|
356 docs|142 tests
|