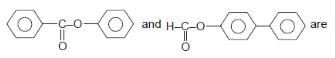KVS PGT Chemistry Mock Test - 2 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test KVS PGT Exam Mock Test Series 2024 - KVS PGT Chemistry Mock Test - 2
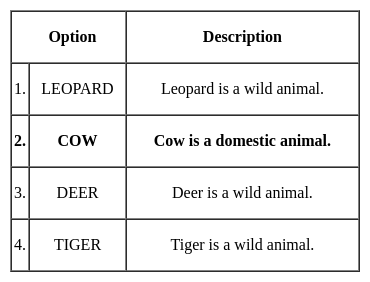
Improve the bracketed part of the sentence with the parts given below.
Q. It is a known fact that some people have (blind faith) in superistitions in our country.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
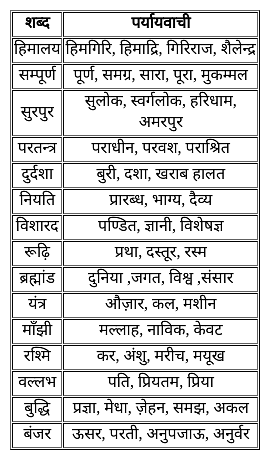
निम्नलिखित में से कौन सा समानार्थी शब्दों का युग्म सही नहीं है?
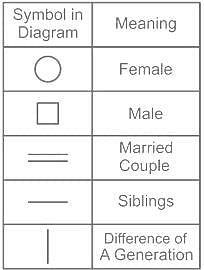
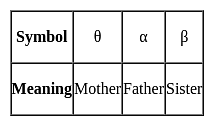
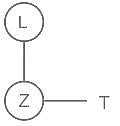
A θ B means A is mother of B, A α B means A is father of B, A β B means A is sister of B. What does L θ Z β T mean?
What is important in ICT supported teaching learning strategies?
Which of the following is not the guideline to enhance questioning skills among children classroom?
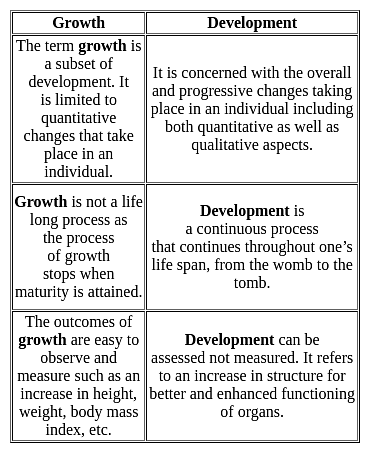
In the context of development, which of the following statement is correct?
I. Development is as much a matter of the child acquiring a culture as it is a process of biological growth.
II. Changes, continuity and stability are not involved in the process of development.
Development is a continuous process, but growth does not continue throughout life, it stops when ______ has been attained.
During electrolysis of acidified water ,O2 gas is formed at the anode. To produce O2 gas at the anode at the rate of 0.224 cc per second at STP,current passed is
Provide the structure of the major organic product which results in the following reaction.
Which reaction, with the following values of ΔH and ΔS at 400 K is spontaneous and endothermic?
Order of the photochemical reaction occurring between hydrogen and chlorine is
Which among the following is adsorbed greatly by activated charcoal?
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The correct statement regarding a chiral compound is
Which of the following represents the decreasing order of van der Waals’ forces in halogens ?
For which of the following reaction the units of rate constant and rate of the reaction are same?
The molal elevation constant is the elevation in boiling point of
In balancing the half-reaction, CN- → CNO-
The number of electrons that must be added is
The dissociation energy of CH4 and C2H6 to convert them into gaseous atoms are 360 and 620 kcal mol-1 respectively. Thus, bond energy of (C—C) bond is
Once the equilibrium is reached under given condition:
Given, ΔfH° of HCI (g) is - 22 . 10 kcal mol-1 and ΔSolutionH° (heat of solution) of HCI (g) is - 17.9 kcal mol-1. Thus, ΔfH ° of Cl- (aq) is
Which major organic product is formed in the following reaction ?