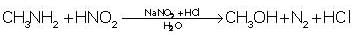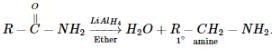KVS PGT Chemistry Mock Test - 5 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test KVS PGT Exam Mock Test Series 2024 - KVS PGT Chemistry Mock Test - 5
दिए गए शब्द युग्म का सही अर्थ ज्ञात कीजिए।
सर्व-शर्व
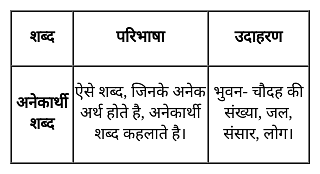
निम्नलिखित प्रश्न में, चार विकल्प दिए गए हैं जिनमें से एक शब्द दिए गए अनेकार्थी शब्द का एक अर्थ नहीं है। उस शब्द का चयन करें।
घन
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Who is popularly known as the father of Evolutionary Biology?
The First Cotton Textile Mill was established in India at
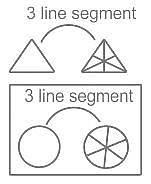
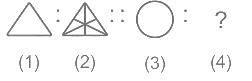
Select the figure that will come next in place of ?.
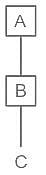
If A is the father of B and B is the father of C, then how is C related to A?
What is the correct order of reactivity of the followings in hydrolysis reaction at elevated temperature?
Which is the most suitable reagent for the following transformation?
Based on the following thermochemical reactions at 298 K and 1 bar
Q. Enthalpy of vaporisation of H2O (l) is
Hot conc. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and nonmetals. Which of the following element is oxidised by conc. H2SO4 into two gaseous products?
Which of the following amine liberates nitrogen gas on reaction with HNO2 ?
Three elements needed for the healthy growth of plants are
Which reaction can be used for the direct conversion of amides into 10 amine ?
The points which shows the position of atoms in a crystal are called as _________.
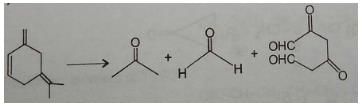
An unsaturated hydrocarbon on jcomplete hydrogenation gives 1-isopropyl-3 methylcyclohexane, after ozonolysis it gives one mole of formaldehyde, one mole of acetone and one mole of 2,4-Dioxohexanedial. The possible structures of the hydrocarbon maybe
The number of radial nodes in 3s and 2p respectively are
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion when freshly prepared FeSO4 solution is added to aqueous solution of
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q.Oxidation number of the Fe in the ring is
At 675 K, H2(g) and CO2(g) react to form CO(g) and H2O(g), Kp for the reaction is 0.16. If a mixture of 0.25 mole of H2(g) and 0.25 mol of CO2 is heated at 675 K, mole% of CO(g) in equilibrium mixture is :
Heat of hydrogenation of ethene is x1 and that of benzene is x2. The resonance energy of benzene is
Replacement of diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by