MCQ (Previous Year Questions) - Isomerism (Level 2) - JEE MCQ
14 Questions MCQ Test - MCQ (Previous Year Questions) - Isomerism (Level 2)
Which of the following compounds will exhibit cis-trans isomerism?
Which of the following compounds exhibits stereoisomerism?
[JEE 2002(Scr.)]
In the given conformation, if C2 is rotated about C2 - C3 bond anticlockwise by an angle of 120° then the conformational obtained is [JEE 2004(Scr.)]
The number of structural isomers for C6H14 is [JEE 2007]
Statement-1: Molecules that are not superimposable on their mirror images are chiral. because
Statement-2: All chiral moleculs have chiral centres. [JEE 2007]
The correct statement(s) about the compuond given below is (are)
The correct statement(s) concerning the structures E, F and G is (are) [JEE 2008]
The correct statement(s) about the compound H3C(OH)HC - CH = CH - CH(OH)CH3(X) is (are)
[JEE 2009]
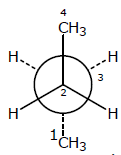
In the Newman projection for 2,2-dimethylbutane [JEE 2010]
X and Y cn respectively be
Amongst the given options, the compound(s) in which all the atoms are in one plane in all the possible conformations (in any), is (are) [JEE 2011]
In Allene (C3H4), the type (s) of hybridisation of the carbon atoms is (are) [JEE 2012]
The number of optically active product obtained from the complete ozonolysis of the given compound is
[JEE 2012]
Which of the following molecules in pure from is (are) unstable at room temperature [JEE 2012]
Which of the given statement(s) about N, O, P and Q with respect to M is (are) correct? [JEE 2012]