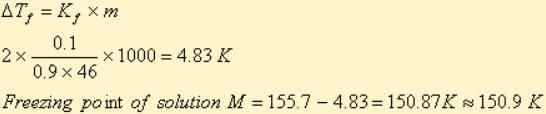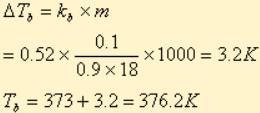MCQ (Previous Year Questions) - Solutions (Level 2) - JEE MCQ
13 Questions MCQ Test - MCQ (Previous Year Questions) - Solutions (Level 2)
The van't Hoff factor for 0.1 M Ba(NO3)2 solution is 2.74. The degree of dissociation is [JEE 1999]
In the depression of freezing point experiment, it is found that [JEE 1999]
(I) The vapour pressure of the solution is less than that of pure solvent.
(II) The vapour pressure of the solution is more than that of pure solvent.
(III) Only solute molecules solidify at the freezing point.
(IV) Only solvent molecules solidify at the freezing point
During depression of freezing point in a solution, the following are in equilibrium [JEE 2003]
A 0.004 M solution of Na2SO4 is isotonic with a 0.010 M solution of glucose at same temperature. The apparent degree of dissociation of Na2SO4 is : [JEE 2004]
The elevation in boiling point, when 13.44 g of freshly prepared CuCl2 are added to one kilogram of water, is [Some useful data, Kb(H2O)= 0.52 kg K mol_1, mol. wt. of CuCl2= 134.4 gm ] [JEE 2005]
When 20 g of naphtholic acid (C11H8O2) is dissolved in 50 g of benzene (Kf = 1.72 K kg mol_1), a freezing point depression of 2 K is observed. The van't Hoff factor (i) is : [JEE 2007]
Properties such as boiling point, freezing point and vapour pressure of a pure solvent change when solute molecules are added to get homogeneous solution. These are called colligative properties. Applications of colligative properties are very useful in day-today life. One of its examples is the use of ethylene glycol and water mixture as anti-freezing liquid in the radiator of automobiles.
A solution M is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture is 0.9.
Given: Freezing point depression constant of water 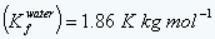
Freezing point depression constant of ethanol 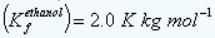
Boiling point elevation constant of 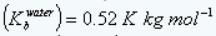
Boiling point elevation constant of ethanol 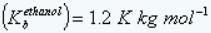
Standard freezing point of water = 273 K
Standard freezing point of ethanol = 155.7 K
Standard boiling point of water = 373 K
Standard boiling point of ethanol = 351.5 K
Vapour pressure of pure water = 32.8 mm Hg
Vapour pressure of pure ethanol = 40 mm Hg
Molecular weight of water = 18 g mol−1
Molecular weight of ethanol = 46 g mol−1
In answering the following questions, consider the solutions to be ideal dilute solutions and solutes to be non-volatile and non-dissociative. [JEE 2008]
The freezing point of the solution M is
The vapour pressure of the solution M is
Water is added to the solution M such that the mole fraction of water in the solution becomes 0.9. The boiling point of this solution is:
The Henry's law constant for the solubility of N2 gas in water at 298 K is 1.0 × 105atm. The mole fraction of N2 in air is 0.8. The number of moles of N2from air dissolved in 10 moles of water at 298 K and 5 atm pressure is : [JEE 2009]
The freezing point (in ºC) of a solution containing 0.1 g of K3[Fe(CN)6](Mol. Wt. 329) in 100 g of water (Kf = 1.86 K kg mol_1) is [JEE 2011]
. For a dilute solution containing 2.5 g of a non-voltile non-electrolyte solute in 100 g of water, the elevation in boiling point at 1 atm pressure is 2ºC. Assuming concentration of solute is much lower than the concentration of solvent, the vapour pressure (mm of Hg) of the solution is (take Kb = 0.76 K kg mol_1) [JEE 2012]
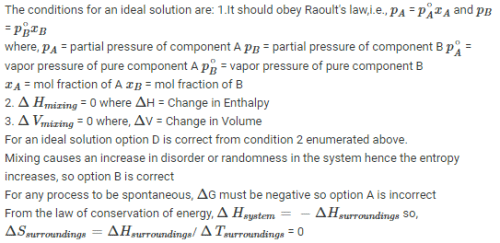
Benzene and naphthalene form an ideal solution at room temeprature. For this process the true statement(s) is (are) [JEE 2013]