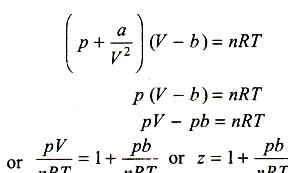MCQ (Previous Year Questions) - States Of Matter (Level 2) - JEE MCQ
10 Questions MCQ Test - MCQ (Previous Year Questions) - States Of Matter (Level 2)
Which one of the following V, T plots represents the behaviour of one mole of an ideal gas at one atmp? [JEE 2002]
Positive deviation from ideal behaviour takes place because of [JEE 2003]
The ratio of the rate of diffusion of helium and methane under identical condition of pressure and temperature will be [JEE 2005]
The given graph represents the variation of Z (compressibility factor = ) versus P, for three real gases A, B and C. Identify the only incorrect statement. [JEE 2006]
Match gases under specific conditions listed in Column I with their properties / laws in Column II. Indicate your answer by darkening the appropriate bubbles of the 4 × 4 matrix given in the ORS. [JEE 2007]
Column I
(A) Hydrogen gas (P = 200 atm, T = 273 K)
(B) Hydrogen gas (P ~ 0, T = 273 K)
(C) CO2 (P = 1 atm, T = 273 K)
(D) Real gas with very large molar volume
Column II
(P) Compressibility factor 1
(Q) Attractive forces are dominant
(R) PV = nRT
(S) P (V - nb) = nRT
A gas described by van der Waals equation [JEE 2008]
For one mole of a van der Waals gas when b = 0 and T = 300 K, the PV vs. l/V plot is shown below. The value of the van der Waals constant a (atm. liter2 mol-2) is [JEE 2012]
A fixed mass `m' of a gas is subjected to transformation of states from K to L to M to N and back to K as shown in the figure.
The succeeding operations that enable this transformation of states are [JEE (ADVANCED)-2013,3/120]
A fixed mass `m' of a gas is subjected to transformation of states from K to L to M to N and back to K as shown in the figure.
The pair of isochoric processes among the transformation of states is : [JEE (ADVANCED)-2013,3/120]
A fixed mass `m' of a gas is subjected to transformation of states from K to L to M to N and back to K as shown in the figure.
Two non-reactive monoatomic ideal gases have their atomic masses in the ratio 2 : 3. The ratio of their partial pressures, when enclosed in a vessel kept at a constant temperature, is 4 : 3. The ratio of their densities is
[JEE (ADVANCED)-2013]