NCERT Based Test: Electronic Configurations & Types of Elements; s, p, d & f-Blocks - NEET MCQ
15 Questions MCQ Test - NCERT Based Test: Electronic Configurations & Types of Elements; s, p, d & f-Blocks
Match the atomic numbers given in column I with the block in which the element is placed in colurim II and mark the appropriate choice.
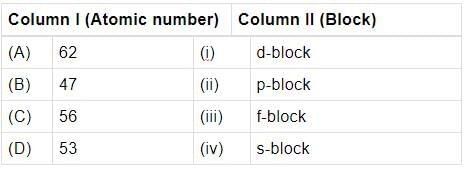

Atomic numbers of few elements are given below. Which of the pairs belongs to s-block?
Which of the following have the same number of electrons in outermost shell?
An element has the electronic configuration
1s2 2s2 2p6 3s2 3p6 3d8 4s2.
What will be its position in.the periodic table?
Fill in the blanks by picking the correct option.
There are _____ groups and _____ periods in the extended form of periodic table. The group, all members of which are in gaseous state under ordinary conditions is _____ group. Most electropositive elements belong to _____ group.
Examples of elements belonging to s, p, d or f-block are given below. Identify the wrong example,
Electronic configurations of few elements are given below. Mark the incorrect match.
Few general names are given along with their valence shell configurations. Mark the incorrect name.
Electronic configuration of four elements is given below. Which of the following does not belong to the same group?
There are two rows of inner transition elements in the periodic table each containing 14 elements. The reason for this may be
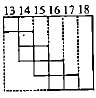
Part of the periodic table showing p-block is depicted below. What are the elements shown in the zig-zag boxes called? What is the nature of the elements outside this boundary on the right side of the table?
The electronic configuration of few elements is given below. Mark the statement which is not correct about these elements.
(i) 1s22s22p63s1
(ii) 1s22s22p5
(iii) 1s22s22p6
(iv) 1s22s22p3
Which of the following is not correct statement for periodic classification of elements?
Indicate the wrong statement on the basis of the periodic table.
Which is the most non-metallic element among the following?



















