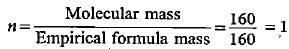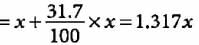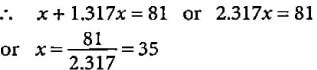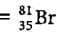NEET Part Test - 1 - NEET MCQ
30 Questions MCQ Test - NEET Part Test - 1
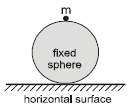
A particle of mass m begins to slide down a fixed smooth sphere from the top as shown. What is its acceleration when it breaks off the sphere?

The height above surface of earth where the value of gravitational acceleration is one fourth of that at surface, will be
A carnot engine is taking 700 cal from source and is rejecting 500 cal to the sink in each cycle. What is the temperature of sink if the source temperature is 150° C .
According to Carnot theorem no heat engine working between two given temperatures of source and sink can be more efficient than a perfectly ___________ engine working between the same two temperatures
Internal energy of a system increases by 60 J when 140 Jof heat is added to the gaseous system. The amount of work done would be:
A body of mass 2kg is dragged on a horizontal surface with a constant speed of 2 m/s. If the coefficient of friction between the body and the surface is 0.2, then find the heat generated in 5 sec.
0.48 g of a sample of a compound containing boron and oxygen contains 0.192 g of boron and 0.288 gof oxygen. What will be the percentage composition of the compound?
Choose the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively and its molecular mass is 160.
A certain metal when irradiated by light (v = 3.2 x 1016 Hz) emits photoelectrons with twice K.E. as did photoelectrons when the same metal is irradiated by light (v = 2.0 x 1016 Hz). The v0 of the metal is
An element with mass number 81 contains 31.7% more neutrons as compared to protons. Find the symbol of the atom
Given
I. C (diamond) + O2(g) → CO2(g) ; ΔH° = - 91.0 kcdl mol-1
II. C(graphite) + O2(g) → CO2(g) ; ΔH° = - 94.0 kcal mol-1
Q. At 298 K, 2.4 kg of carbon (diamond) is converted into graphite form. Thus, entropy change is
The solubility product expression for tin(II) hydroxide, Sn(OH)2, is
The [Ag+(aq)] = 10-5 in a solution .The [Cl–(aq)] to precipitate AgCl having Ksp of 1.8×10-10 M2 is — M
Consider the following equilibrium in a closed container
N2O4 (g)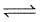 2NO2(g)
2NO2(g)
At a fixed temperature, the volume of a reaction container is halved. For this change, which of the following statements holds true regarding the equilibrium constant (Kp) and degree of dissociation (α) ?
The discovery of which of the following group of elements gave a death blow to the Newlands Law -
Consider the following statement (i)-(iii) and select the correct option stating which ones are true (T) and which one are false (F)
(i) Non Keratinised stratified squamous epithelium covers moist surfaces like buccal cavity
(ii) Fibroblasts store fat in adipose tissue
(iii) Urinary bladder is lined by a stratified epithelium.
In the given diagram of a section of hyaline cartilage, the different parts have been indicated by alphabets. Choose the answer in which these alphabets correctly match the parts they indicate.
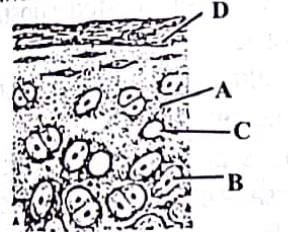
Given is the diagrammatic sketch of a certain type of connective tissue. Identify the parts labeled A, B, C and D and select the right option about them
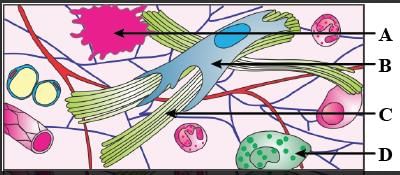
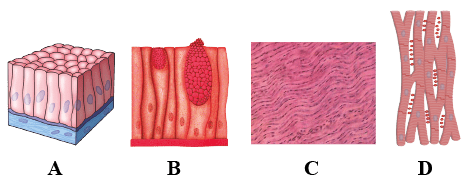
The four figures (A, B, C and D) given below represent four different types of animals tissues. Which one of these is correctly identified in the given options along with its correct location and function?
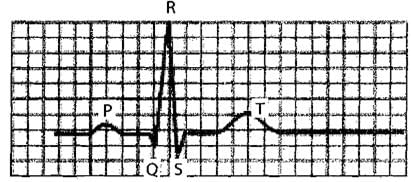
The given figure is the ECG of a normal human. Which one of its components is correctly interpreted below
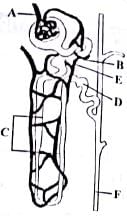
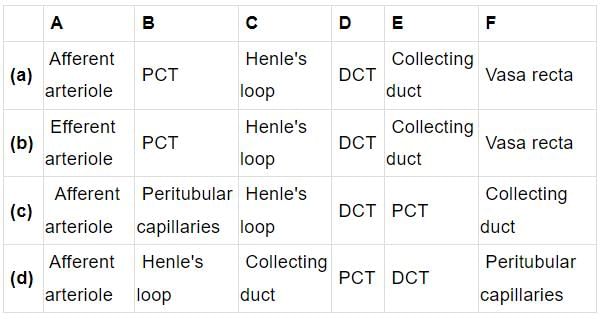
Select the option that correctly identifies the parts lateral from A to F in the given figure of nephron.
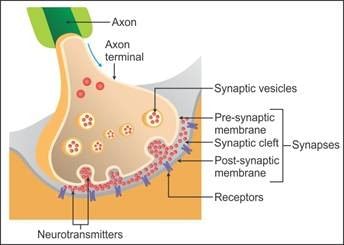
A diagram showing the axon terminal and synapse is given. Identify correctly at least two of A-D:
The signal transduction of steroid hormone across cell is through
Direction:
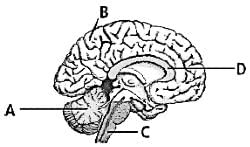
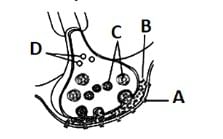
Which of the following functions is performed by the labelled 'C' in the given figure?
Read the following statements :
i. Every 100 ml of deoxygenated blood delivers about 4 ml of CO2 to the alveoli.
ii. 30% - 40% of CO2 is transported to the alveoli as carbamino-haemoglobin.
iii. RBCs contain a very high concentration of the enzyme, carbonic anhydrase and minute quantities of the same is present in the plasma too
Which of the statements given above is/are correct about the process of respiration?
i. Breathing involves the exchange of atmospheric air and CO2-rich alveolar air.
ii. Oxygen and carbon dioxide diffuse across the alveolar membrane during respiration.
iii. Blood transports only carbon dioxide from tissues to the lungs.
iv. Cells utilize oxygen for anabolic reactions, releasing carbon dioxide as a byproduct.