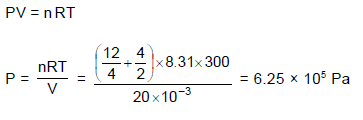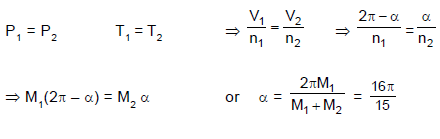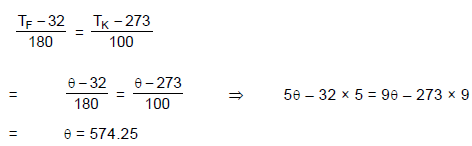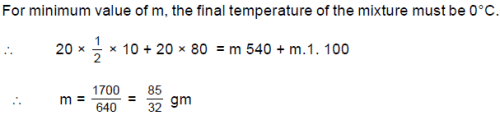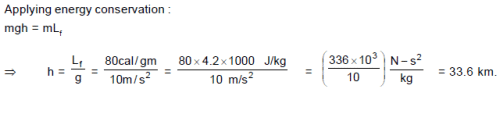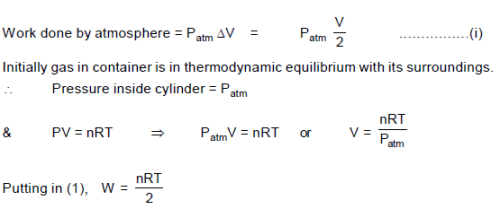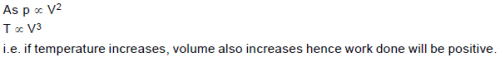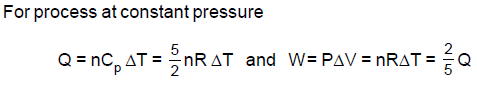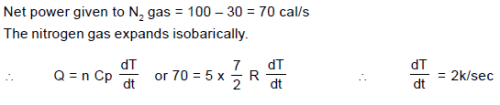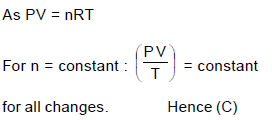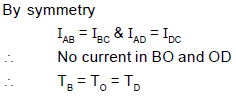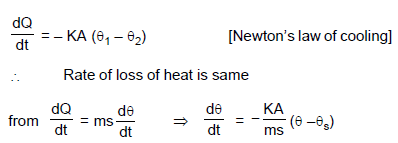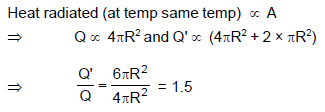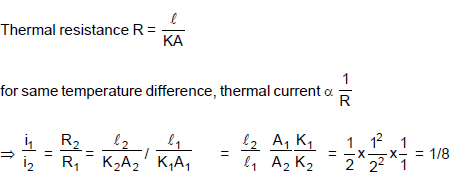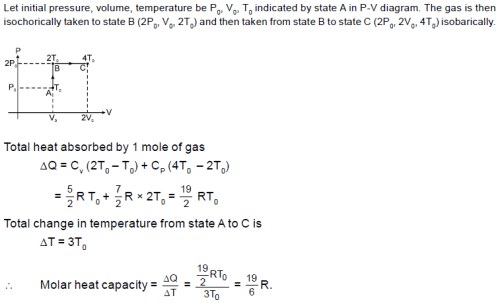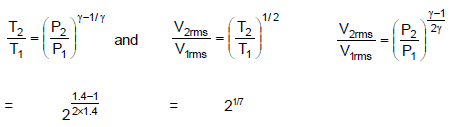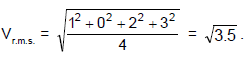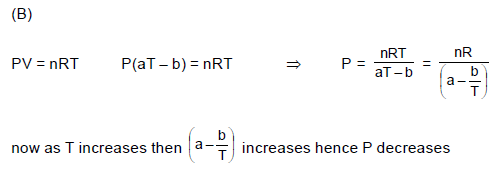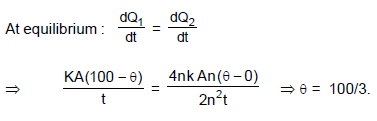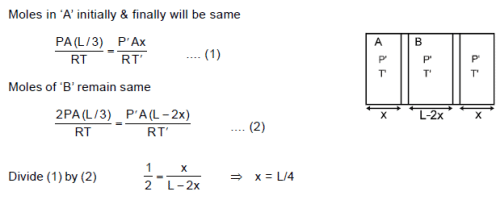Part Test- 5 (JEE Main 2018) - JEE MCQ
30 Questions MCQ Test - Part Test- 5 (JEE Main 2018)
A gas mixture consists of 2 moles of oxygen and 4 moles of argon at temperature T. Neglecting all vibrational modes, the total internal energy of the system is:
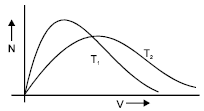
Maxwell’s velocity distribution curve is given for two different temperatures. For the given curves.

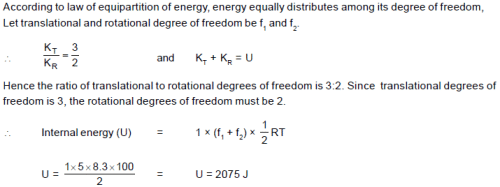
The ratio of translational and rotational kinetic energies at 100 K temperature is 3 : 2. Then the internal energy of one mole gas at that temperature is[R = 8.3 J/mol-K]
12 gm He and 4 gm H2 is filled in a container of volume 20 litre maintained at temperature 300 K. The pressure of the mixture is nearly :
Which of the following will have maximum total kinetic energy at temperature 300 K.
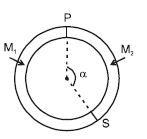
A ring shaped tube contains two ideal gases with equal masses and atomic mass numbers M1 = 32 and M2 = 28. The gases are separated by one fixed partition P and another movable conducting partition S which can move freely without friction inside the ring. The angle a as shown in the figure in equilibrium is:
In an experiment the speeds of any five molecules of an ideal gas are recorded. The experiment is repeated N times where N is very large. The average of recorded values, is :
Temperature at which Fahrenheit and Kelvin pair of scales give the same reading will be:
20 gm ice at –10 ºC is mixed with m gm steam at 100 ºC. The minimum value of m so that finally all ice and steam converts into water is : (Use sice=0.5 cal/gmºC,swater =1 cal/gmºC,L (melting)=80 cal/gm and L (vaporization)=540 cal/gm)
An ice block at 0°C is dropped from height ‘h’ above the ground. What should be the value of ‘h’ so that it melts completely by the time it reaches the bottom assuming the loss of whole gravitational potential energy is used as heat by the ice ? [Given : Lf = 80 cal/gm]
n moles of a gas filled in a container at temperature T is in thermodynamic equilibrium initially. If the gas is compressed slowly and isothermally to half its initial volume the work done by the atmosphere on the piston is:
In a process the pressure of an ideal gas is proportional to square of the volume of the gas. If the temperature of the gas increases in this process, then work done by this gas:
A vessel contains an ideal monoatomic gas which expands at constant pressure, when heat Q is given to it. Then the work done in expansion is:
5 moles of Nitrogen gas is enclosed in an adiabatic cylindrical vessel. The piston itself is a rigid light cylindrical container containing 3 moles of Helium gas. There is a heater which gives out a power 100 cal/sec to the nitrogen gas. A power of 30 cal /sec is transferred to Helium through the bottom surface of the piston. The rate of increment of temperature of the nitrogen gas assuming that the piston moves slowly:
The gas law PV/T = constant for a given amount of a gas is true for :
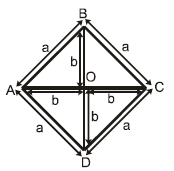
All the rods have same conductance ‘K’ and same area of cross section S. If ends A and C are maintained at temperature 2T0 and T0 respectively then which of the following is/are correct:
A hollow copper sphere and a hollow copper cube, of same surface area and negligible thickness, are filled with warm water of same temperature and placed in an enclosure of constant temperature, a few degrees below that of the bodies. Then in the beginning :
Two identical solid spheres have the same temperature. One of the sphere is cut into two identical pieces. The intact sphere radiates an energy Q during a given small time interval. During the same interval, the two hemispheres radiate a total energy Q'. The ratio Q'/Q is equal to :
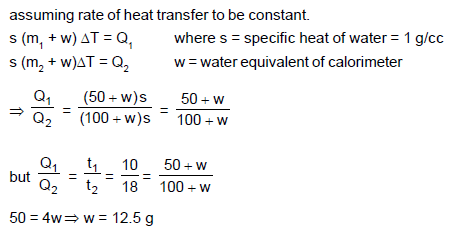
A calorimeter contains 50 g of water at 50°C. The temperature falls to 45°C in 10 minutes. When the calorimeter contains 100 g of water at 50°C, it takes 18 minutes for the temperature to become 45°C. then the water equivalent of the calorimeter is :
Heat is flowing through two cylindrical rods made of same materials whose ends are maintained at similar temperatures. If diameters of the rods are in ratio 1 : 2 and lengths in ratio 2 : 1, then the ratio of thermal current through them in steady state is :
A balloon containing an ideal gas has a volume of 10 litre and temperature of 17ºC. If it is heated slowly to 75ºC, the work done by the gas inside the balloon is (neglect elasticity of the balloon and take atmospheric pressure as 105 Pa)
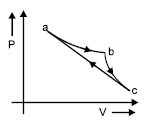
In the P-V diagram shown. The gas does 5 J of work in isothermal process a b and 4 J in adiabatic process b c. What will be the change in internal energy of the gas in straight path c to a?
A monoatomic ideal gas is filled in a nonconducting container. The gas can be compressed by a movable nonconducting piston. The gas is compressed slowly to 12.5% of its initial volume. The percentage increase in the temperature of the gas is
A diatomic ideal gas is heated at constant volume until the pressure is doubled and again heated at constant pressure until volume is doubled. The average molar heat capacity for whole process is:
The ratio of final root mean square velocity to initial root mean square velocity of nitrogen molecules if nitrogen gas is compressed adiabatically from a pressure of one atmosphere to a pressure of two atmosphere is :
Four particles have velocities 1, 0, 2, 3 m/s. The root mean square velocity of the particles is: (in m/s)
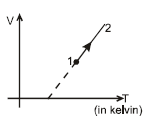
V–T diagram for a process of a given mass of ideal gas is as shown in the figure. During the process pressure of gas.
A slab X of thickness ‘t’, thermal conductivity ‘K’ and area of cross-section ‘A’ is placed in thermal contact with another slab Y which is 2n2 times thicker,4n times conductive and having n times larger cross section area. If the outside face of X is maintained at 100°C, the outside face of Y at 0°C,then the temperature of the junction θ is represented by the graph (n > 0) :
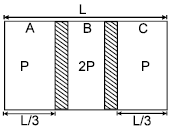
Two conducting movable smooth pistons are kept inside a non conducting, adiabatic container with initial positions as shown. Gas is present in the three parts A, B & C having initial pressures as shown. Now the pistons are released. Then the final equilibrium position length of part A will be