Periodic Properties - IIT JAM MCQ
30 Questions MCQ Test - Periodic Properties
Which of the following element has the highest first ionization energy:
Electron gain enthalpy and ionization energy of an atom are –a and +b eV respectively. The electronegativity of that atom on Mulliken scale is given by:
Which process is different from other on the basis of enthalpy change?
Which of the following should be correct for Z1 and Z2 in the following two processes
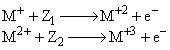
Which of the following process is used in extractive metallurgy of magnesium?
Solutions of equal strength of XOH and QOH are prepared. The I.P.’s of X and Q are 5.1 and 13.0 eV respectively, whereas their E.N’s are 0.9 and 3.2 respectively. Using the information spot the incorrect conclusion:
In which of the following pairs, both the hydrides are not of the same type:
The dipole moment of LiH is 1.964 × 10–29 C-m and the interatomic distance between Li and H in this molecule is 1.596 Å. What is the percent ionic character in LiH:
Two elements X and Y have following electronic configuration:
X: 1s22s22p63s23p64s2
Y: 1s22s22p63s23p5
The expected compound formed by combination of X and Y will be expressed as:
In the commercial electrochemical process for aluminium extraction, the electrolyte used as:
In the process of extraction of gold, 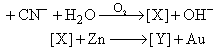
Identify the complexes [X] and [Y]
Which of the following magnetic moment value will correspond to highest ionization energy from Mn species:
Which of the following metal is the strongest reducing agent in gas phase:
The electron affinity of oxygen family in the periodic table, follows the order:
Identify the correct order of acidic strengths of CO2, CuO, CaO, H2O
Arrange the following elements in increasing order of their ionization energy.
Al, Ga, In, Tl
The intermolecular van der Waals’ potential is inversely proportional to r6. The corresponding force is proportional to:














