Retro (Past 13 Year) IIT- JEE Advanced (Chemical Kinetics) - JEE MCQ
10 Questions MCQ Test - Retro (Past 13 Year) IIT- JEE Advanced (Chemical Kinetics)
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
For the elementary reaction M → N, the rate of disappearance of M increases by a factor of 8 upon doubling the concentration of M. The order of the reaction with respect to M is
(2014 Adv., Only One Option Correct Type)
In the reaction , 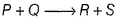 the time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure . The over all order of the reaction is
the time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure . The over all order of the reaction is
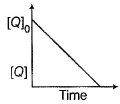
(2013 Adv., Only One Option Correct Type)
An organic compound undergoes first order decomposition . The time taken for its decomposition to 1/8 and 1/10 of its initial concentration are t 1/8 and t 1/10 respectively. What is the value of 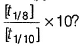 (log10 2 = 0.3)
(log10 2 = 0.3)
(2012, Integer Type)
For the first order reaction,
(2011, One or More than One Options Correct Type)
Plots showing the variation of the rate constant (k) with temperature (T) are given below. The plot that follows Arrhenius equation is
(2010, Only One Option Correct Type)
The concentration of R in the reaction R → P was measured as a function of time and the following data is obtained :
Q.
The order of the reaction is
(2010 , inte ger Type)
For a first order reaction , the temperature (T) dependent rate constant (k) was found to follow the equation
The pre-exponential factor A and the activation energy Ea, respectively, are
(2009, Only One Option Correct Type)
Under the same reaction conditions, in itial concentration of 1.386 mol dm -3 of a substance becomes half in 40 s and 20 s through first order and zero order kinetics respectively. Ratio of the rate constants for first order (k1) and zero order (k0) of the reaction is
(2008, Only One Option Correct Type)
Consider a reaction products. When concentration of both the reactants G and H is doubled, the rate increases by eight times . Flowever, when concentration of G is doubled keeping the concentration of H fixed, the rate is doubled. The overall order of the reaction is
(2007, Only One Option Correct Type)
Which one of the following statements is incorrect about order of reaction?
(2005, Only One Option Correct Type)














