Retro (Past 13 Year) IIT-JEE Advanced (Electrochemistry) - JEE MCQ
18 Questions MCQ Test - Retro (Past 13 Year) IIT-JEE Advanced (Electrochemistry)
In a galvanic cell, the salt bridge
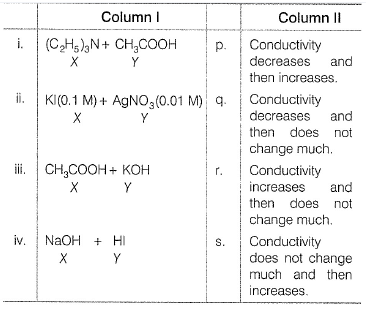
An aqueous solution of X is added slowly to an aqueous solution of Y as shown in Column I. The variation in conductivity of these reactions is given in Column II. Match Column I with Column II and select the correct answer using the codes given below.

The standard reduction potential data at 25°C is given below.
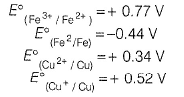
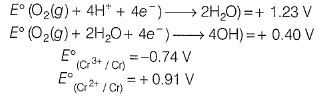
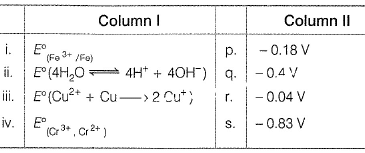
Match E° of the rebox pair in Column I with the values given in Column II and select the correct answer using the codes given below.

(2013 Adv., Matching Type)
Passage for Q. Nos. (4-5)
The electrochemical cell shown below is a concentration cell. M | M2+ (saturated solution of a sparingly soluble salt, MX2) | | M2+ (0.001 mol dm-3) |M The emf of the cell depends on the difference in concentration of M2+ ions at the two electrodes. The emf of the cell at 298K is 0.059 V.
Q.
The solubility product (Ksp ; mol3dm-9) of MX2 at 298 K based on the information available the given concentration cell is (take 2.303x R x 298 / F= 0.059 V)
The electrochemical cell shown below is a concentration cell. M | M2+ (saturated solution of a sparingly soluble salt, MX2) | | M2+ (0.001 mol dm-3) |M The emf of the cell depends on the difference in concentration of M2+ ions at the two electrodes. The emf of the cell at 298K is 0.059 V.
Q.
The value of ΔG (kJ mol-1) for the given cell is (take 1 F= 96500 C mol-1)
Consider the following cell reaction,
At [Fe2+] = 10-3 M, p(O2) = 0.1 atm and pH = 3, the cell potential at 25°C is
(2011, Only One Option Correct Type)
AgNO3 (aqueous) was added to an aqueous KCI solution gradually and the conductivity of the solution was measured. The plot of conductance (A) versus the volume of AgNO3 is
(2011, Only One Option Correct Type)
Passage for Q. N os. (8-9)
The concentration of potassium ions inside a biological cell is at least twenty times higher than the outside. The resulting potential difference across the cell is important in several processes such as transmission of nerve impulses and maintaining the ion balance. A simple model for such a concentration cell involving a metal M is M(s) | M+ (aq 0.05 molar) | | M+ (ag; 1 molar) | M(s).
For the above electrolytic cell the magnitude of the cell potential | Ecell | = 70 mV.
Q.
For the above cell
The concentration of potassium ions inside a biological cell is at least twenty times higher than the outside. The resulting potential difference across the cell is important in several processes such as transmission of nerve impulses and maintaining the ion balance. A simple model for such a concentration cell involving a metal M is M(s) | M+ (aq 0.05 molar) | | M+ (ag; 1 molar) | M(s).
For the above electrolytic cell the magnitude of the cell potential | Ecell | = 70 mV.
Q.
If the 0.05 molar solution of M+ is replaced by a 0.0025 molar M+ solution, then the magnitude of the cell potential would be
For the reduction of NO3- ion in an aqueous solution E° is + 0.96 V. Values of E° for some metal ions are given below
The pair(s) of metals that is(are) oxidised by NO3- in aqueous solution is(are)
(2009, One or More than One Options Correct Type)
Electrolysis of dilute aqueous NaCI solution was carried out by passing 10 mA current. The time required to liberate 0.01 mole of H2 gas at the cathode is (1 F = 96500 C mol-1)
(2008,Only One Option Correct Type)
Passage for Q. Nos. (12-13)
Redox reaction play a vital role in chemistry and biology. The values of standard redox potential (E°) of two half-cell reactions decide which way the reaction is expected to proceed. A simple example is a Daniell cell in which zinc goes into solution and copper gets deposited. Given below are a set of half-cell reactions (acidic medium) along with their E° (V with respect to normal hydrogen electrode) values.
Q.
Among the following, identify the correct statement.
Redox reaction play a vital role in chemistry and biology. The values of standard redox potential (E°) of two half-cell reactions decide which way the reaction is expected to proceed. A simple example is a Daniell cell in which zinc goes into solution and copper gets deposited. Given below are a set of half-cell reactions (acidic medium) along with their E° (V with respect to normal hydrogen electrode) values.
Q.
While Fe3+ is stable, Mn3+ is not stable in acid solution because
Passage for Q. Nos. (14-16)
Tollen’s reagent is used for the detection of aldehydes. When a solution of AgNO3 is added to glucose with NH4OH, then gluconic acid is formed.
Q.
Find In K of this reaction
Tollen’s reagent is used for the detection of aldehydes. When a solution of AgNO3 is added to glucose with NH4OH, then gluconic acid is formed.
Q.
When ammonia is added to the solution, pH is raised to 11. Which half-cell reaction is affected by pH and by how much?
Tollen’s reagent is used for the detection of aldehydes. When a solution of AgNO3 is added to glucose with NH4OH, then gluconic acid is formed.
Q.
Ammonia is always added in this reaction. Which of the following must be incorrect?
We have taken a saturated solution of AgBr, Ksp is 12 x 10-14 If 10-7 M of AgNO3 are added to 1 L of this solution, find conductivity (specific conductance) of this solution in terms of 10-7 Sm-1 units.
(2006, Subjective Type)
The half-cell reactions for rusting of iron are:
ΔG° (in kJ) for the reaction is
(2005, Only One Option Correct Type)



















