Retro (Past 13 Years) IIT-JEE Advanced (Chemical Bonding) - JEE MCQ
11 Questions MCQ Test - Retro (Past 13 Years) IIT-JEE Advanced (Chemical Bonding)
Assuming 2s-2p mixing is not operative, the paramagnetic species among the following is
(2014 Adv., Single Option Correct Type)
Hydrogen bonding plays a central role in the following phenomena
(2014 Adv., One or More than Option Correct Type)
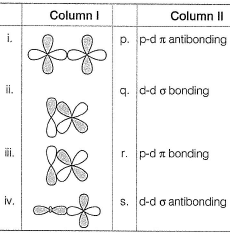
Match the orbital overlap figures shown in Column I with the description given in Column II and select the correct answer using the codes given below the columns.

Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is
(2010, Single Option Correct Type)
The species having pyramidal shape is
(2010, Single Option Correct Type)
Match each of the diatomic molecules in Column I with its property/properties in Column II.

Hyperconjugation involves overlap of the following orbitals
(2008, Single Option Correct Type)
The species having bond order different from that in CO is
(2007, Single Option Correct Type)
Among the following, the paramagnetic compound is
(2007, Single Option Correct Type)
Match the reactions in Column I with nature of the reactions/type of the products in Column II.
Which of the following contains maximum number of lone pairs on the central atom?
(2005, Single Option Correct Type)














