Retro (Past 13 Years) IIT - JEE Advanced (States Of Matter) - JEE MCQ
12 Questions MCQ Test - Retro (Past 13 Years) IIT - JEE Advanced (States Of Matter)
Retro JEE Advanced (Compilation of Last 10 Years Questions)
IIT JEE (Main & Advanced) past year questions with solutions & answer key
Retro IIT JEE (Main and Advanced) have questions from previous/past (10 to 13 years) papers of IIT JEE (Main and Advanced) with solutions,
The following set of questions contain previous/past (10 to 13 years) questions of IIT JEE (Main and Advanced) of Chapter "State of Matter" with solutions
Passage For Q. Nos. 1-2
X and Y are two volatile liquids with molar weights of 10 g mol-1 and 40 g mol-1 respectively. Two cotton plugs, one soaked in X and the other soaked in V, are simultaneously placed at the ends of a tube of length L = 24cm, as shown in the figure.
The tube is filled with an inert gas at 1 atm pressure and a temperature of 300 K. Vapours of X and Y react to form a product which is first observed at a distance d cm from the plug soaked in X. Take X and V to have equal molecular diameters and assume ideal behaviour for the inert gas and the two vapours.
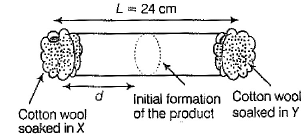
Q. The value of d in cm (shown in the figure), as estimated from Graham’s law, is
(2014 Adv, Comprehension Type)
IIT JEE (Main & Advanced) past year questions with solutions & answer key
Retro IIT JEE (Main and Advanced) have questions from previous/past (10 to 13 years) papers of IIT JEE (Main and Advanced) with solutions,
The following set of questions contain previous/past (10 to 13 years) questions of IIT JEE (Main and Advanced) of Chapter "State of Matter" with solutions
The tube is filled with an inert gas at 1 atm pressure and a temperature of 300 K. Vapours of X and Y react to form a product which is first observed at a distance d cm from the plug soaked in X. Take X and V to have equal molecular diameters and assume ideal behaviour for the inert gas and the two vapours.
X and Y are two volatile liquids with molar weights of 10 g mol-1 and 40 g mol-1 respectively. Two cotton plugs, one soaked in X and the other soaked in V, are simultaneously placed at the ends of a tube of length L = 24cm, as shown in the figure.
The tube is filled with an inert gas at 1 atm pressure and a temperature of 300 K. Vapours of X and Y react to form a product which is first observed at a distance d cm from the plug soaked in X. Take X and V to have equal molecular diameters and assume ideal behaviour for the inert gas and the two vapours.

Q. The experimental value of d is found to be smaller than the estimate obtained using Graham's law. This due to
The tube is filled with an inert gas at 1 atm pressure and a temperature of 300 K. Vapours of X and Y react to form a product which is first observed at a distance d cm from the plug soaked in X. Take X and V to have equal molecular diameters and assume ideal behaviour for the inert gas and the two vapours.
If the value of Avogadro number is 6.023 x 1023 mol-1 and the value of Boltzmann constant is 1.380 x 10-23 JK-1, then the number of significant digits in the calculated value of the universal gas constant is
(2014, Integer Type)
For one mole of a van der Waals' gas when b = 0 and T = 300 K, the pV vs 1/V plot is shown below. The value of the van der Waals' constant a (atm L mol-2)
According to kinetic theory of gases
(2011, Single Option Correct)
To an evacuated vessel with movable piston under external pressure of 1 atm, 0.1 mole of He and 1.0 mole of an unknown compound (vapour pressure 0.68 atm at 0°C) are introduced. Considering the ideal gas behaviour, the total volume (in litre of the gases Of 0°C is close to)
(2011, Integer Type)
The term that corrects for the attractive forces present in a real gas in the van der Waals' equation is
(2009, Single Option Correct)
At 400 K, the root main square (rms) speed of a gas X (molecular weight = 40) is equal to the most probable speed of gas y at 60 K. The molecular weight of the gas y is
(2009, Integer Type)
A gas described by van der Waals' equation
(2003, Single Option Correct)
Match the gases under specified conditions listed in Column I with their properties/laws in Column lI.
The given graph represent the variations of Z (compressibility factor versus p, for three real gases A, B and C.
Identify the only incorrect statement.
(2006, Single Option Correct)
If helium and methane are allowed to diffuse out of the container under the similar conditions of temperature and pressure, then the ratio of rate of diffusion of helium to methane is
(2005, Single Option Correct)




















