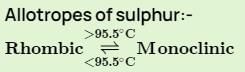Sample Test: Chemistry - NEET MCQ
20 Questions MCQ Test - Sample Test: Chemistry
The ratio of velocity of electron in 2nd to that in 4th excited state of Be3+ will be
Monomer of which of the following has atoms of only two different elements
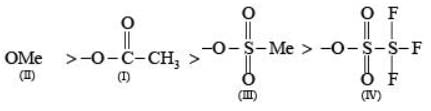
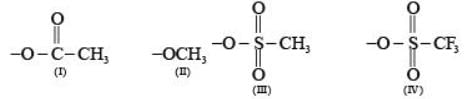
Arrange the following compounds in the order of leaving group ability
The strongest acid among the following aromatic compounds is
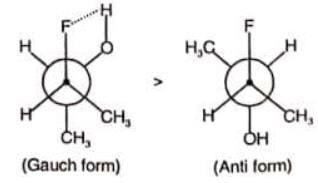
In which of the following, gauche form is more stable than anti form of conformers?
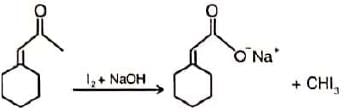
Which of the following derivatives of benzene would undergo hydrolysis most readily with aq. KOH?
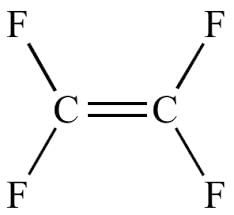
The compound showing maximum number of geometrical isomers is
Aqueous CuSO4 solution is blue in colour. Which colour is absorbed by it ?
Which of the following elements can form square planar fluoride?
Transition temperature of rhombic sulphur to monoclinic sulphur is
During electrophoresis of purple of cassius, the colloidal particles will
In a reaction, 64.2 M of reactant becomes 32.1 M in 20 second. After 30 second from the start of the reaction 16.05 M is left. The value of rate constant will be
Correct comparison of ΔTb of the following (aqueous solution) is
1. 0.1 N NaCl
2. 0.1 N CaCl2
3. 0.1 N AlCl3
4. 0.1 N AI2 (SO)4