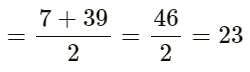Science Olympiad Test: Periodic Classification of Elements- 2 - Class 10 MCQ
15 Questions MCQ Test - Science Olympiad Test: Periodic Classification of Elements- 2
The element with atomic number 14 is hard and forms acidic oxide and a covalent halide. To which of the following categories does the element belong?
What type of oxide would Eka-aluminium form?
Which of the following does not increase while moving down the group of the periodic table?
Which of the following elements will form an acidic oxide?
Arrange the following elements in the order of the their increasing non-metallic character Li, O, C, Be, F
Elements A, B and C Constitute a Dobereiner’s triad. If the atomic mass of element A is 7 and that of element C is 39, then what is the atomic mass of element B?
X and Y are two elements having similar properties which obey Newland’s Law of Octaves. The minimum and maximum number of elements in between X and Y, respectively are
The two elements for which Mendeleev left blank places in his original periodic table were
Which of the following statement(s) about the modern periodic table are incorrect
(i) The elements in the modern periodic table are arranged on the basis of their decreasing atomic number.
(ii) Isotopes are placed in adjoining group(s) in the periodic table
(iii) The elements in the modern period table are arranged on the basis of their increasing atomic masses.
(iv) The elements in the modern periodic table are arranged on the basis of their increasing atomic number.
The elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 respectively. Which pair of elements belong to the same group?
Which of the following statements about the modern period table is correct?
Which one of the following elements exhibit maximum number of valence electrons?
Which of the following gives the correct increasing order of the atomic radii of O, F and N?
Which of the following are the characteristics of isotopes of an element?
(i) Isotopes of an element have same atomic masses
(ii) Isotopes of an element have same atomic number
(iii) Isotopes of an element show same chemical properties
(iv) Isotopes of an element show same physical properties
Three elements B, Si and Ge are