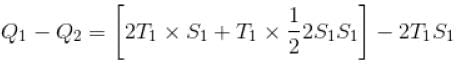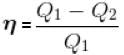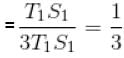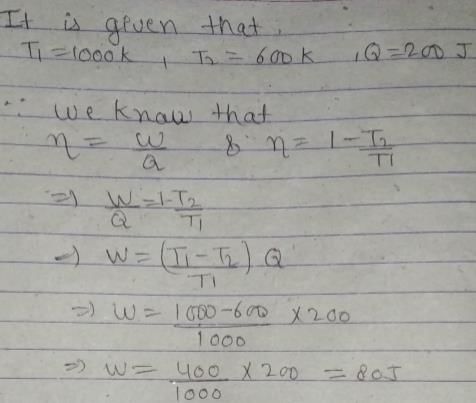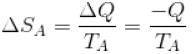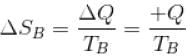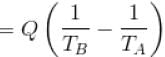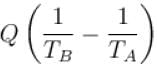Second Law Of Thermodynamics MCQ Level – 2 (Part - 2) - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Second Law Of Thermodynamics MCQ Level – 2 (Part - 2)
Consider a system of N atoms of an ideal gas of type A at temperature T and volume V. It is kept in diffusive contact with another system of N atoms of another ideal gas of type B at the same temperature T and volume V. Once the combined system reaches equilibrium.
Select one:
Select one:
A heat pump uses 300 J of work to remove 400 J of heat from the low-temperature reservoir. How much heat is delivered to a higher temperature reservoir?
For the thermodynamics process shown in the figure, corresponding T-S diagram is
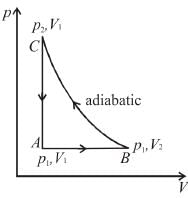
Select one:

Select one:
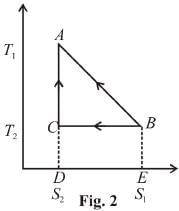
The T–S diagram of two cycles for the operation of an engine are shown in figure below.
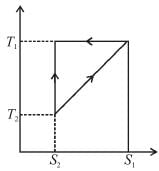

The numerical values of the parameters T1 , T2 , S1 and S2 in the two figures are the same, then which cycle has greater efficiency?
Select one:
An electric current of 3A flows through a resistance of 10 ohm. It is being cooled by running water and is kept at temperature 300K, change in entropy per second of the resistance :
Select one:
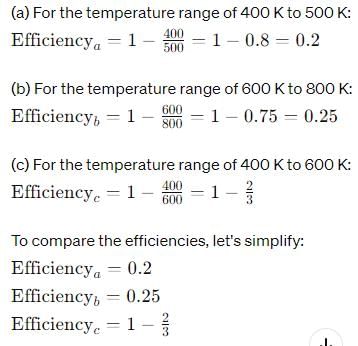
Out of three Carnot engines, operating between reservoir temperature of (a) 400 and 500 K (b) 600 and 800 K (c) 400 and 600 K, which has the greater thermal efficiency?
A reversible engine cyclic is shown in the following T–S diagram. The efficiency of the engine is :
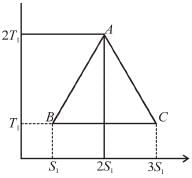
Select one:
An engine absorbs heat at temperature of 1000 K and rejects heat at 600 K. If the engine operates at maximum possible efficiency, the amount of work performed by the engine for 200 J heat input is
Select one:
An amount of heat Q is transferred from a heat reservoir at temperature TA to another heat reservoir at temperature TB. What is the change in the entropy Δs of the combined system?
Select one:
The difference in entropy between a state of volume Vi and a state of volume Vf (temperature and number of molecules remaining constant) is equal to :
Select one: