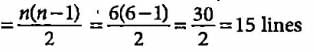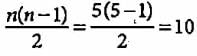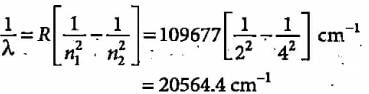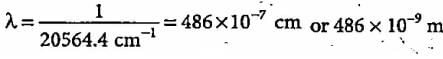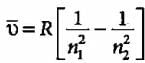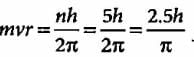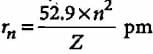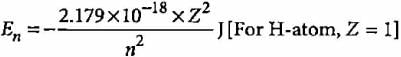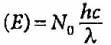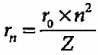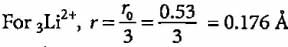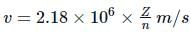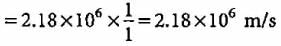Test: Bohr's Model for Hydrogen Atom - NEET MCQ
20 Questions MCQ Test - Test: Bohr's Model for Hydrogen Atom
The electron in Bohr’s model of hydrogen atom is pictured as revolving around the nucleus in order for it to
Given below are the spectral lines for an atom of hydrogen. Mark the lines which are not correctly matched with the value of n1 and n2?


What is the maximum number of emission lines when the excited electron of a hydrogen atom in n = 6 drops to ground state?
What is the maximum number of emission lines obtained when the excited electron of a H atom in n = 5 drops to the ground state?
What is the colour corresponding to the wavelength of light emitted when the electron in a hydrogen atom undergoes transition from n = 4 to n = 2?
_____series of lines are the only lines in hydrogen spectrum which appear in the visible region.
An electron in excited hydrogen atom falls from fifth energy level to second energy level. In which of the following regions, the spectrum line will be observed and is part of which series of the atomic, spectrum?
The third line of the Balmer series in the emission spectrum of the hydrogen atom is due to the transition from the
The angular momentum of an electron in a given stationary state can be expressed as 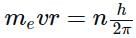 .
.
Based on this expression an electron can move only in those orbits for which its angular momentum is?
According to Bohr’s theory, the angular momentum of an electron in 5th orbit is
The radius of the stationary state which is also called Bohr radius is given by the expression rn = n2a0 where the value of a0 is
If the radius of first Bohr orbit is x pm, then the radius of the third orbit would be
What does the negative electronic energy (negative sign for all values of energy) for hydrogen atom means?
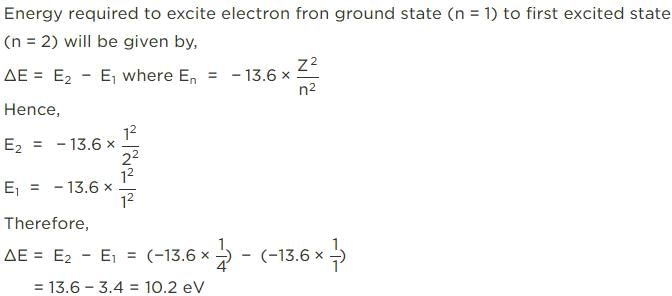
If the ionisation energy of hydrogen atom is 13.6 eV, the energy required to excite it from ground state to the next higher state is approximately
Bohr’s theory can also be applied to the ions like
According to Bohr’s theory, the electronic energy of H-atom in Bohr’s orbit is given by
Which of the following is not correctly matched?
What is the trend of energy of Bohr’s orbits?
The radius of hydrogen atom in ground state is 0.53 Å. What will be the radius of 3Li2+ in the ground state?
What is the velocity of electron present in first Bohr orbit of hydrogen atom?