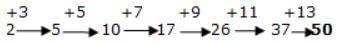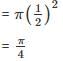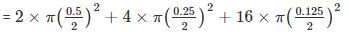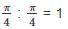Test: CSIR-NET Chemical Sciences Mock Test - 8 - UGC NET MCQ
30 Questions MCQ Test - Test: CSIR-NET Chemical Sciences Mock Test - 8
The sugar unit present in DNA is derived from-
The next term in the series
2, 5, 10, 17, 26, 37, ___?___ is:
2, 5, 10, 17, 26, 37, ___?___ is:
At present a person is 4 time older than his son and is 3 years older than his wife. After 3 years the age of the son will be 15 years. The age of the person’s wife after 5 years will be:
Assertion (A): Informal fallacies have a defect because of the poor form of the argument.
Reasoning (R): Informal fallacies have good form in the argument itself, but there is a defect in the content.
The ratio of two numbers a and b is 3:7. After adding 9 to each number, the ratio becomes 9:17. The numbers a and b are:
Single-grain density and bulk density of four varieties of grains are given in the table. If we take 1 kg of each of these, which one will have the largest volume?
Suppose there are 6 non-stop flights from Chennai to Mumbai in the morning and 4 non-stop flights from Mumbai to Goa in the evening. In how many ways can one fly from Chennai to Goa via Mumbai using these flights in a day?
The present age of a father is square of the age of his son. After six years, the age of the father would be 3(1/2) times the age of the son. The present age of the father is
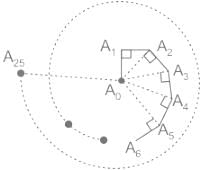
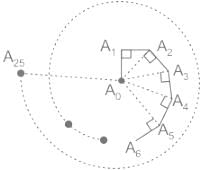
A bug moves in a straight line from a point A0 to a point A1 1 cm away. It then turns by an angle of 90° and moves straight another 1 cm to reach a point A2. Then it moves 1 cm perpendicular to the line joining A0 to A2 to a point A3. It continues this way as shown, moving 1cm every time till it reaches a point A25. What is the straight line distance from A0 to A25?
There are 150 vehicles in a parking place. Each vehicle is either a bike or a car, and is either red or green. Sixty vehicles are red, and 100 vehicles are cars. If there are 20 green bikes, how many red cars are there?
The number of times the minute hand and the hour hand, in a clock, are exactly above each other (i.e. angle between them is zero) from 1 am of a day to 1 am on the next day is
The shaded circles having diameter of 1, 0.5, 0.25 and 0.125 cm are inside squares of side 1 cm as shown in the figure. The ratio of shaded area in A and B is one.

The ratio of shaded area in C and D would be
Each person in a group of teachers and students is given the same number of chocolates as the number of students. If 4 more students are added then in order to have the same number of chocolates per person as earlier, 28 more chocolates are needed. The total number of students now is
Methane is at the rate 50 moles is supplied to a reactor with air at the rate 1000 moles, the reactor leaves O2, N2, CO2 and H2O, what is the percentage of N2 in products?
Change in phase is an example of which process?
On which factor the rate constant of a reaction does not depend upon?
How many chiral stereoisomers can be drawn for CH3CHFCHFCH(CH3)2?
Which one of the following statements best describes the enthalpy change of a reaction?
Methane is at the rate 50 moles is supplied to a reactor with air at the rate 1000 moles, the reactor leaves O2, N2, CO2 and H2O, what is the percentage of H2O in products?
Acetone reacts with HCN to form a cyanohydrin. It is an example of which type of reaction?
How much nitrogen must be added to a 20% nitrogen solution to obtain 100 Kg of 40% nitrogen solution?
Which of the following statement is incorrect about nucleophiles?
Which of the following is the definition of a pair of diastereomers?
1 mole glucose reacts completely with excess air, if 25 liter of bone dry air is produced at 27oC and 5 atm, what is the humidity?
The rate of reaction that does not involve gases, is not dependent on:
What is the pressure of 1 mole of an ideal gas at 27ºC occupying 1 Liter of volume?
Which of the following compounds can exhibit geometrical isomerism?
The rate of flow in stream with 50% O2 and 50% CO2 is 10 mole/hr, the contained was initially filled with 10 mole of O2, what will be the percentage of O2 in product stream?