Chemistry Exam > Chemistry Tests > Inorganic Chemistry > Test: Chemical Bonding- 2 - Chemistry MCQ
Test: Chemical Bonding- 2 - Chemistry MCQ
Test Description
30 Questions MCQ Test Inorganic Chemistry - Test: Chemical Bonding- 2
Test: Chemical Bonding- 2 for Chemistry 2025 is part of Inorganic Chemistry preparation. The Test: Chemical Bonding- 2 questions and answers have been
prepared according to the Chemistry exam syllabus.The Test: Chemical Bonding- 2 MCQs are made for Chemistry 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Chemical Bonding- 2 below.
Solutions of Test: Chemical Bonding- 2 questions in English are available as part of our Inorganic Chemistry for Chemistry & Test: Chemical Bonding- 2 solutions in
Hindi for Inorganic Chemistry course. Download more important topics, notes, lectures and mock
test series for Chemistry Exam by signing up for free. Attempt Test: Chemical Bonding- 2 | 30 questions in 90 minutes | Mock test for Chemistry preparation | Free important questions MCQ to study Inorganic Chemistry for Chemistry Exam | Download free PDF with solutions
Test: Chemical Bonding- 2 - Question 2
Among the following pairs in which the two species are not isostructural is:
Test: Chemical Bonding- 2 - Question 3
Which of the following set of ions/ molecules is isoelectric and structural?
Detailed Solution for Test: Chemical Bonding- 2 - Question 3
Test: Chemical Bonding- 2 - Question 4
In allene (C3H4), the type(s) of hybridisation of the carbon atoms is (are)
Detailed Solution for Test: Chemical Bonding- 2 - Question 4
Test: Chemical Bonding- 2 - Question 5
The atom that form discrete polyatomic molecule in its elemental state are:
Detailed Solution for Test: Chemical Bonding- 2 - Question 5
Detailed Solution for Test: Chemical Bonding- 2 - Question 7
Test: Chemical Bonding- 2 - Question 8
Which of the following species has two non bonded electron pairs on the central atom:
Detailed Solution for Test: Chemical Bonding- 2 - Question 8
Test: Chemical Bonding- 2 - Question 9
The shape and expected hybridization of BrO3- and HOCl are:
Test: Chemical Bonding- 2 - Question 11
The hybridization of atomic orbital of nitrogen in NO2+, NO3- and NH4+ are:
Detailed Solution for Test: Chemical Bonding- 2 - Question 11
Detailed Solution for Test: Chemical Bonding- 2 - Question 12
Detailed Solution for Test: Chemical Bonding- 2 - Question 13
Test: Chemical Bonding- 2 - Question 15
Covalent-molecules are usually held in a crystal structure by
Detailed Solution for Test: Chemical Bonding- 2 - Question 15
Detailed Solution for Test: Chemical Bonding- 2 - Question 17
Detailed Solution for Test: Chemical Bonding- 2 - Question 19
Detailed Solution for Test: Chemical Bonding- 2 - Question 23
Test: Chemical Bonding- 2 - Question 26
In octahedral structure the pair of ‘d’ orbitals invo lved is:
Test: Chemical Bonding- 2 - Question 28
Hybridization state of boron and oxygen in boric acid is:
Detailed Solution for Test: Chemical Bonding- 2 - Question 28
Detailed Solution for Test: Chemical Bonding- 2 - Question 30
|
50 videos|92 docs|41 tests
|
Information about Test: Chemical Bonding- 2 Page
In this test you can find the Exam questions for Test: Chemical Bonding- 2 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Chemical Bonding- 2, EduRev gives you an ample number of Online tests for practice



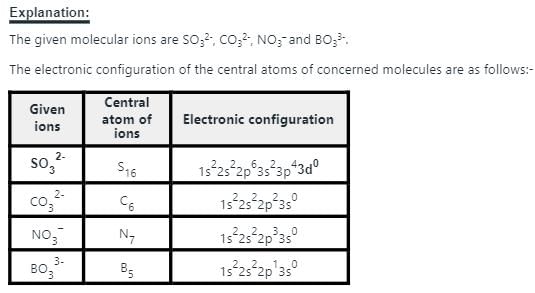
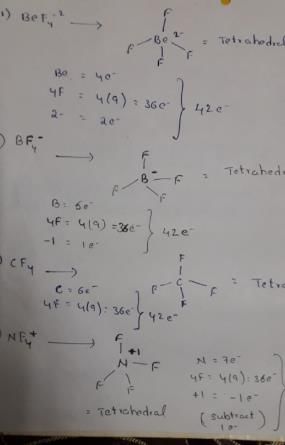 Isoelectronic species are those have same number of electrons.
Isoelectronic species are those have same number of electrons.


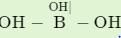 ; has a network structure in which boron is trigonal having sp2 and each oxygen atom is tetrahedral having sp3-hybridization with two lone pair of electrons on oxygen.
; has a network structure in which boron is trigonal having sp2 and each oxygen atom is tetrahedral having sp3-hybridization with two lone pair of electrons on oxygen.










