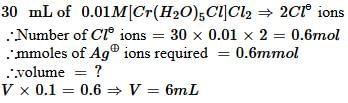Test: Coordination Chemistry- 3 - Chemistry MCQ
30 Questions MCQ Test - Test: Coordination Chemistry- 3
The correct order of wavelength (λmax) of the halide to metal charge-transfer band of [Co(NH3)5Cl]2+ (I), [Co(NH3)5Br]2+ (II) and [Co(NH3)5I]2+ (III), is
The ground state term symbols for high spin d5s1 and d5 configurations, respectively are:
The volume (in mL) of 0.1 M AgNO3 required for complex precipitation of chloride ions present in 30 mL of 0.01 M solution of [Cr(H2O)5Cl]Cl2, as silver chloride is close to
The strongest ligand in spectro–chemical series is:
[NiCl2(PPh3)2] is paramagnetic with μeff = 2.9 BM. All the four ligands are monodentate. The geometry of the molecule is:
A solution containing 2.675 g of CoCl3 · 6NH3 (molar mass = 267.5 g mol–1) is passed through a cation exchanger. The chloride ions obtained in solution were treated with excess of AgNO3 to give 4.78 g of AgCl (molar mass = 143.5 g mol–1). The formula of the complex is
(At. Mass of Ag = 108 u)
For which of the following ions is the colour in aqueous solution not caused by any d–d transition?
The cyano complex that exhibit highest value of paramagnetism is:
An excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetraaquachromium(III) chloride. The number of moles of AgCl precipitated would be
The pair of compounds having metals in their highest oxidation state is
Correct relat ionship between pairing energy (P) and C.F.S.E (∆0) in complex ion [Ir(H2O)6]3+ is:
In which of the following pairs, both the complexes have the same hybridization?
The value of CFSE (∆0) for complexes given below follow the order:
(I) [Co(NH3)6]3+
(II) [Rh(NH3)6]3+
(III) [Ir(NH3)6]3+
Cr3+ form for complexes with four different ligands which are [Cr(Cl)6]3–,[Cr(H2O)6]3+, [Cr(NH3)6]3+ and [Cr(CN)6]3–. The order of CFSE (∆0) in these complexes is in the order:
The d–orbital, which are stabilized in an octahedral magnetic field, are:
Total number of geometrical isomers for the complex [RhCl(CO)(PPh3)(NH3)]is
Which of the following is correct arrangement of ligand in terms of the Dq values of their complexes with any particular ‘hard’ metal ion:
The extent of crystal field splitting in octahedral complexes of the given metal with particular weak field ligand are:
A magnetic moment of 1.73 BM will be shown by one among the following
Consider the following two reactions:
According to given information the correct statement (s) is/are:
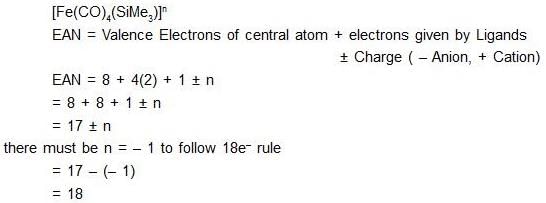
The value of n for the complex [Fe(CO)4(SiMe3)]n satisfying the 18-electron rule is
Select correct statement (s) regarding octahedron complex having CFSE = –1.2∆0.