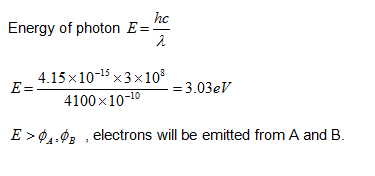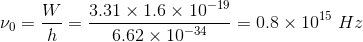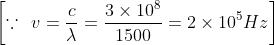Test: Dual Nature of Radiation and Matter - 2 - CUET Humanities MCQ
10 Questions MCQ Test Agriculture Practice Tests: CUET Preparation - Test: Dual Nature of Radiation and Matter - 2
Work function of a metal is 5.2 × 10–18. Its threshold wavelength will be
Which one of the following graphs represents the variation of maximum kinetic energy (Ek) of the emitted electrons with frequency (v) in photoelectric effect correctly?
The work functions of metals A, B and C are 1.92 eV, 2.0 eV and 5 eV, respectively. According to Einstein's equation, which metal(s) will emit the photoelectrons for a radiation of wavelength 4100 Å?
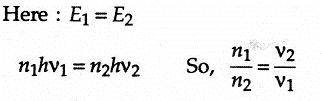
There are n1 photons of frequency v1 in a beam of light. In an equally energetic beam, there are n2 photons of frequency v2. The correct relation is
A metal has work function 3.31 eV. It is illuminated by light of wavelength 3 x 10-7m.What is the threshold frequency for photoelectric emission? ( Take h = 6.62 ×10-34 J s)
Threshold wavelength for photoelectric emission, from a metal surface, is 5200 Å. Photoelectrons will be emitted when this surface is illuminated with monochromatic radiation from
A radio transmitter operates on a wavelength of 1500 m at a power of 400 kilowatt. The energy of the radio photon (in joules) is
A metal whose work function is 3.31 eV is illuminated by light of wavelength 3 10-7 m. What is the threshold frequency for photoelectric emission? (Take h = 6.62 x 10-34 Js)
A light having wavelength 300 nm falls on a metal surface. Work function of metal is 2.54 eV. What is the stopping potential?
Relation between wavelength of photon and electron of same energy is