Test: Fuel Gas Analysis - 1 - Mechanical Engineering MCQ
20 Questions MCQ Test - Test: Fuel Gas Analysis - 1
The shift conversion reaction taking place during water gas manufacture is given by
In a pulverised-fuel-fired large power boiler, then heat transfer from the burning fuel to the walls of the furnace is
Match List I with List II and select the correct answer:

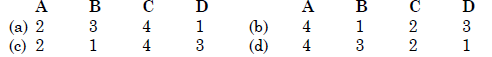
Bomb calorimeter is used to determine the calorific value of
[IES-2003]
Volumetric analysis of sample of dry products -of combustion gave the following results: CO2 = 10% CO = 1% O2 = 8% N2 = 81%
Their proportions by weight will be
solid or liquid fuel can be determined, as the water vapour formed is carried away by the exhaust gases
.Reason (R): The lower calorific value of a fuel is the net value of heat available found by subtracting the latent head of the water formed and carried away by exhaust gas from the higher calorific value.
The calorific value determined by the bomb calorimeter is
Which one of the following gaseous fuels does not have different higherand lower calorific values?
Consider the following statements:
The difference between higher and lower heating values of the fuels is due to
1. Heat carried by steam from the moisture content of fuel.
2. Sensible heat carried away by the flue gases.
3. Heat carried away be steam from the combustion of hydrogen in the fuel.
4. Heat lost by radiation On these statements
Consider the following:
Carbon Carbon-monoxide
Hydrogen, Sulphur
What is the amount of oxygen (in kg) required for complete combustion ofeach one of the above respectively?
In Combustion process, the effect of dissociation is to
The amount of CO2 produced by 1 kg of carbon on complete combustion is
Methane burns with stoichiometric quantity of air. The air/fuel ratio by weight is
The mass of air required for complete combustion of unit mass of fuel canalways be calculated from the formula, where C, H, O and S are inpercentage.
One mole of hydrogen is burnt with chemically correct quantity of air andcooled to N.T.P The change in volume in mole is
Match List I with List II and select the correct answer.
Consider the following statements:
1. Pulverised fuel gives high and controlled burning rate
2. Insufficient air causes excessive smoking of exhaust
3. Excess air is provided to control the flue gas temperature
4. Effect of sulphur in fuel is to give high heat transfer rate.Which of these statements are correct?
Coal fired power plant boilers manufactured in India generally use:
Consider the following statements regarding the fluidized bed combustionboilers:
1. The combustion temperatures are low, around 900oC.
2. The formation of oxides of nitrogen is low.
3. It removes sulphur from coal during combustion process.
4. It requires high quality of coal as fuel.Which of these statements are correct?
Which one of the following statements is not correct? In a fluidized-bedboiler?














