Test: Hydrogen & Hydrides (Old NCERT) - JEE MCQ
20 Questions MCQ Test - Test: Hydrogen & Hydrides (Old NCERT)
Out of NH3, H2O and HF, which has the highest magnitude of Hydrogen bonding:
Since the isotopes have the same electronic configuration, they have the same
The physical properties of isotopes differ due to:
Reaction of granulated zinc with dil HCl results in formation of:
Electron-rich hydrides has excess electrons that are present as
Hydrogen has tendency to gain one electron to acquire helium configuration, in this respect it resembles:
NaH when added to water produces a large amount of energy. The hydride will be:
Terrestrial hydrogen contains deuterium mostly in the form of:
Element that is found abundantly in the universe and is the principal element of solar atmosphere is:
Of all the isotopes of hydrogen which one is highly radioactive:
The sum of number of neutrons and protons in tritium is:
Dihydrogen under certain reaction conditions combines with all elements except:


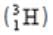 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons. 











