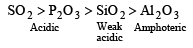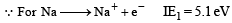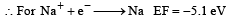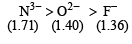Test: JEE Main 35 Year PYQs- Classification of Elements & Periodicity in Properties - JEE MCQ
15 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Classification of Elements & Periodicity in Properties
According to the Periodic Law of elements, the variation in properties of elements is related to their [2003]
Which one of the following is an amphoteric oxide ?
Which one of the following ions has the highest value of ionic radius ? [2004]
Among Al2O3, SiO2, P2O3 and SO2 the correct order of acid strength is [2004]
The formation of the oxide i on O2-(g) requires first an exothermic and then an endothermic step as shown below
O(g) + e- = O-(g) ΔHº =-142 kJmol-1 [2004]
O-(g) + e- = O2-(g) ΔHº= 844 kJmol-1
This is because
Which of the following oxides is amphoteric in character?
In which of the following arrangements, the order is NOT according to the property indicated against it?
Following statements regarding the periodic trends of chemical reactivity of the alkali metals and the halogens are given. Which of these statements gives the correct picture?
In Which of the following arrangement, the given sequence is not strictly according to the property indicated against it ?
The correct sequence which shows decreasing order of the ionic radii of the elements is [2010]
Which one of the following orders presen ts the correct sequence of the increasing basic nature of the given oxides? [2011]
The increasing order of the ionic radii of th e given isoelectronic species is : [2012]
Wh ich of the following represents the corr ect order of increasing first ionization enthalpy for Ca, Ba, S, Se and Ar ? [JEE M 2013]
The first ionisation potential of Na is 5.1 eV. The value of electron gain enthalpy of Na+ will be : [JEE M 2013]
The ionic radii (in Å) of N3–, O2– and F– are respectively : [JEE M 2015]