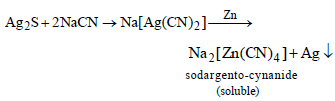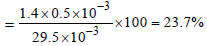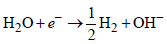Test: JEE Main 35 Year PYQs- General Principles & Processes of Isolation of Elements - JEE MCQ
10 Questions MCQ Test - Test: JEE Main 35 Year PYQs- General Principles & Processes of Isolation of Elements
Aluminium is extracted by the electrolysis of [2002]
The metal extracted by leaching with a cyanide is [2002]
Which one of the following ores is best concentrated byfroth-flotation method ? [2004]
During the process of electrolytic refining of copper, some metals present as impurity settle as ‘anode mud’. These are [2005]
Which of the following factors is of no significance forroasting sulphide ores to the oxides and not subjecting thesulphide ores to carbon reduction directly? [2008]
29.5 mg of an organic compound containing nitrogen wasdigested according to Kjeldahl’s method and the evolvedammonia was absorbed in 20 mL of 0.1 M HCl solution. Theexcess of the acid required 15 mL of 0.1 M NaOH solutionfor complete neutralization. The percentage of nitrogen inthe compound is [2010]
Which method of purification is represented by the following equation ? [2012]
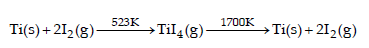
The metal that cannot be obtained by electrolysis of anaqueous solution of its salts is: [JEE M 2014]
In the context of the Hall - Heroult process for the extraction of Al, which of the following statements is false ?[JEE M 2015]
Which one of the following ores is best concentrated byfroth floatation method? [JEE M 2016]