Test: JEE Main 35 Year PYQs- Haloalkanes & Haloarene- 2 - JEE MCQ
18 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Haloalkanes & Haloarene- 2
Chlorobenzene can be prepared by reacting aniline with : (1984 - 1 Mark)
The reaction of toluene with chlorine in presence of ferricchloride gives predominantly : (1986 - 1 Mark)
The reaction conditions leading to the best yields of C2H5Cl are : (1986 - 1 Mark)
n-Propyl bromide on treatment with ethanolic potassiumhydroxide produces (1987 - 1 Mark)
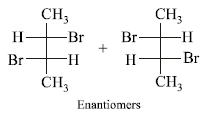
The number of structural and configurational isomers of abromo compound, C5H9Br, formed by the addition of HBr to 2-pentyne respectively are (1988 - 1 Mark)
1-Chlorobutane on reaction with alcoholic potash gives (1991 - 1 Mark)
The chief reaction product of reaction between n-butane and bromine at 130ºC is : (1995 S)
Isobutyl magnesium bromide with dry ether and ethyl alcohol gives : (1995S)
(CH3)3 CMgCl on reaction with D2O produces :
A solution of (+) –2–chloro–2–phenylethane in tolueneracemises slowly in the presence of small amount of SbCl5,due to the formation of (1999 - 2 Marks)
Identify the set of reagent / reaction conditions 'X' and 'Y' in the following set of transformations (2002S)
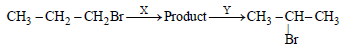
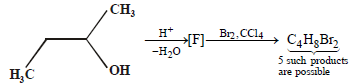
How many structures for F are possible? (2003S)
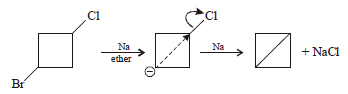
What would be the product formed when 1-bromo-3- chlorocyclobutane reacts with two equivalents of metallic
sodium in ether? (2005 S)
When phenyl magnesium bromide reacts with tert -butanol, the product would be (2005S)
The reagent(s) for the following conversion,
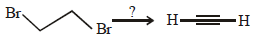
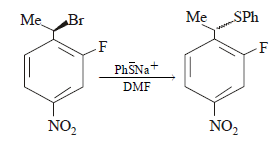
The major product of the following reaction is – (2008)
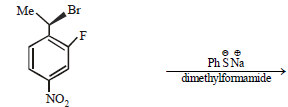
Aryl halides are less reactive towards nucleophilicsubstitution reaction as compared to alkyl halides due to :(1990 - 1 Mark)
Benzyl chloride (C6H5CH2Cl) can be prepared from toluene by chlorination with (1998 - 2 Marks)


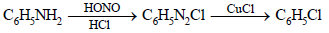

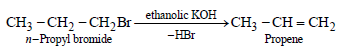
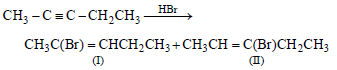
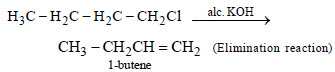
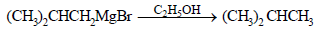
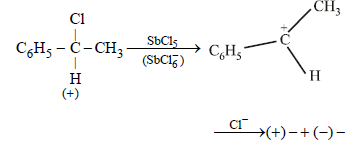
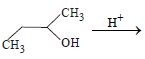
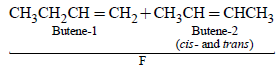
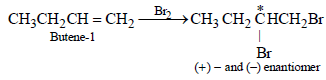
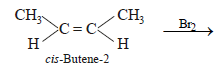
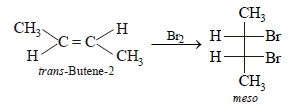
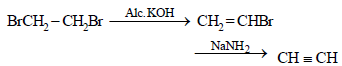
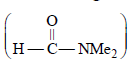 is a highly polar aprotic solvent.
is a highly polar aprotic solvent.