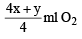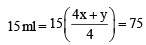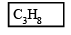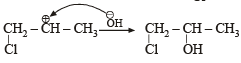Test: JEE Main 35 Year PYQs- Hydrocarbons - JEE MCQ
24 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Hydrocarbons
Which of these will not react with acetylene? [2002]
What is the product when acetylene reacts with hypochlorous acid? [2002]
On mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane.This alkane could be [2003]
2-Methylbutane on reacting with bromine in the presence of sunlight gives mainly [2005]
Butene-1 may be converted to butane by reaction with
Reaction of one molecule of HBr with one molecule of 1, 3-butadiene at 40°C gives predominantly [2005]
Of the five isomeric hexanes, the isomer which can give two monochlorinated compounds is [2005]
Acid catalyzed hydration of alkenes except ethene leads to the formation of
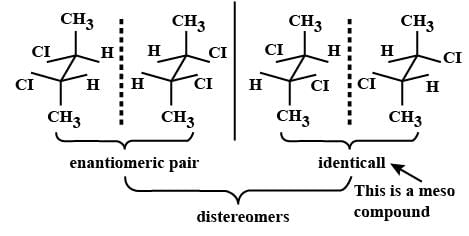
Which types of isomerism is shown by 2, 3-dichlorobutane? [2005]
The compound formed as a result of oxidation of ethyl benzene by KMnO4 is [2007]
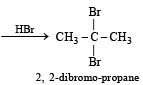
Which of the following reactions will yield 2, 2-dibromopropane? [2007]
The reaction of toluene with Cl2 in presence of FeCl3 gives predominantly [2007]
Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is diazotised and then heated wth cuprous bromide. The reaction mixture so formed contans [2008]
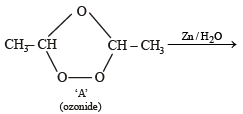
In the following sequence of reactions, the alkene affords the compound ‘B’
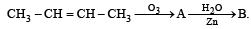
The compound B is [2008]
The hydrocarbon which can react with sodium in liquid ammonia is [2008]
The treatment of CH3MgX with CH3C ≡ C-H produces [2008]
One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44 u. The alkene is[2010]
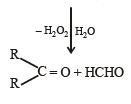
Ozonolysis of an organic compound gives formaldehyde as one of the products. This confirms the presence of : [2011]
Which branched chain isomer of the hydrocarbon with molecular mass 72u gives only one isomer of mono substituted alkyl halide ? [2012]
2-Hexyne gives trans-2-Hexene on treatment with :
Which compound would give 5 - keto - 2 - methylhexanal upon ozonolysis ? [JEE M 2015]
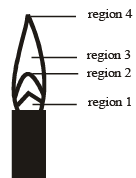
The hottest region of Bunsen flame shown in the figure below is : [JEE M 2016]
At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air containing 20% O2 by volume for complete combustion. After combustion the gases occupy 330 mL.Assuming that the water formed is in liquid form and the volumes were measured at the same temperature and pressure, the formula of the hydrocarbon is: [JEE M 2016]
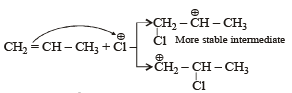
The reaction of propene with HOCl (Cl2 + H2O) proceeds through the intermediate: [JEE M 2016]


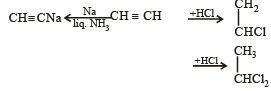

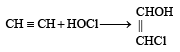
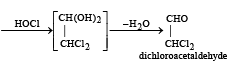

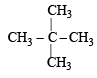
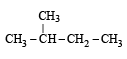
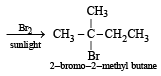
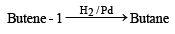
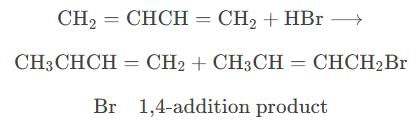
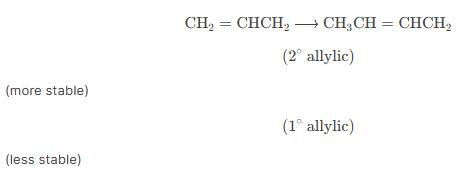
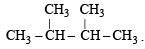 Since it contains only two types of H-atoms hence it will give only two mono chlorinated compounds viz.
Since it contains only two types of H-atoms hence it will give only two mono chlorinated compounds viz. 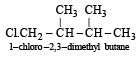 and
and 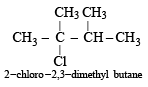
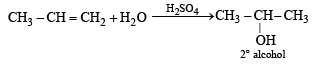
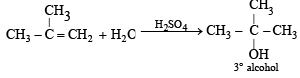
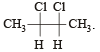
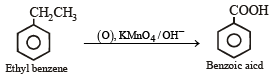
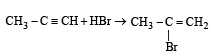
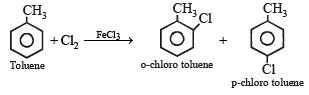
 group whichis o-, p- directing group so on nitration of toluene the – NO2 group will occupy o-, p- positions.
group whichis o-, p- directing group so on nitration of toluene the – NO2 group will occupy o-, p- positions.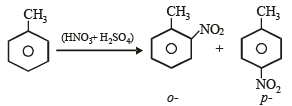
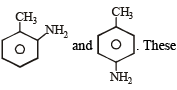 anilines when diazotized and then treated with CuBr forms o-, p- bromotoluenes.
anilines when diazotized and then treated with CuBr forms o-, p- bromotoluenes.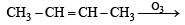
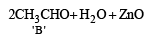
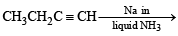
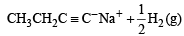
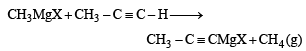
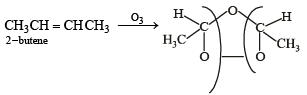
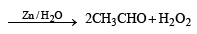
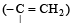 in order to give formaldehyde as one of the product.
in order to give formaldehyde as one of the product.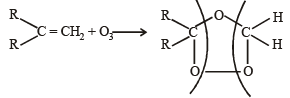
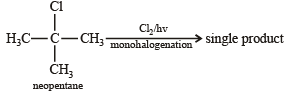
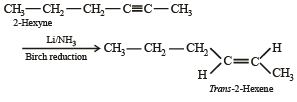
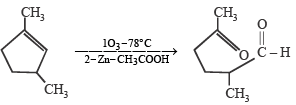
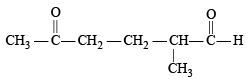
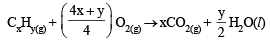
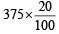 = 75 ml
= 75 ml