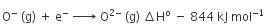Test: JEE Previous Year Questions- Classification of Elements & Periodicity in Properties - JEE MCQ
24 Questions MCQ Test - Test: JEE Previous Year Questions- Classification of Elements & Periodicity in Properties
Which is the correct order of ionic sizes (At. No. : Ce = 58, Sn = 50, Yb = 70 and Lu = 71) [AIEEE-2002]
Ce3+, La3+, Pm3+ and Yb3+ have ionic radii in the increasing order as - [AIEEE-2002]
According to the Periodic Law of elements, the Variation in properties of elements is related to their ?
[AIEEE-2003]
The reduction in atomic size with increase in atomic number is a characteristic of elements of - [AIEEE-2003]
Which one of the following groups represent a collection of isoelectronic species ?
(At. no. Cs = 55, Br = 35) [AIEEE-2003]
The atomic numbers of vanadium (V). Chromium (Cr), manganese (Mn) and iron (Fe) respectively 23, 24, 25 and 26. Which one of these may be expected to have the higher second ionization enthalpy ?
[AIEEE-2003]
Which one of the following sets of ions represents the collection of isoelectronic species ? [AIEEE-2004]
Which one of the following ions has the highest value of ionic radius ? [AIEEE-2004]
Among Al2O3, SiO2, P2O3 and SO2 the correct order of acid strength is :
[AIEEE-2004]
The formation of the oxide ion requires first an exothermic and then an endothermic step as shown below:
O(g) + e- = O-(g) ΔH° = -142 kJ mol-1
O-(g) + e- = O2-(g) ΔH° = 844 kJ mol-1
This is because of :
[AIEEE-2004]
In which of the following arrangements the order is NOT according to the property indicated against it?
[AIEEE-2005]
Which of the following oxides is amphoteric in character ? [AIEEE-2005]
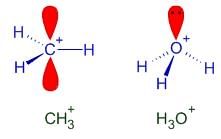
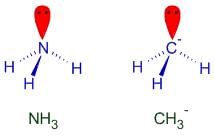
Pick out the isoelectronic structure from the following : [AIEEE-2005]
I. +CH3
II. H3O+
III. NH3
IV. CH3-
The lanthanide contraction is responsible for the fact that [AIEEE-2005]
Which of the following factors may be regarded as the main cause the lanthanide contraction ?
[AIEEE-2005]
The increasing order of the first ionization enthalpies of the elements B, P, S and F (lowest first) is–
[AIEEE-2006]
Which one of the following sets of ions represents a collection of isoelectronic species ? [AIEEE-2006]
lanthanoid contraction is caused due to - [AIEEE-2006]
Which one of the following constitutes a group of the isoelectronic species? [AIEEE-2008]
In which of the following arrangements, the sequence is not strictly according to the property written against it ?
[AIEEE-2009]
The correct sequence which shows decreasing order of the ionic radii of the elements is [AIEEE-2010]
Which one of the following order represents the correct sequence of the increasing basic nature of the given oxides ?
[AIEEE-2011]
The outer electron configuration of Gd (Atomic No : 64 is : [AIEEE-2006]
The increasing order of the ionic radii of the given isoelectronic species is: