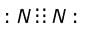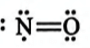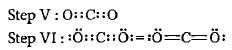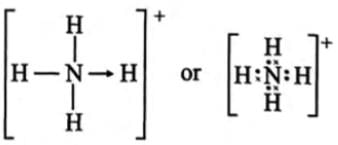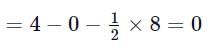Test: Kossel-Lewis Approach to Chemical Bonding (NCERT) - NEET MCQ
10 Questions MCQ Test NCERTs at Fingertips: Textbooks, Tests & Solutions - Test: Kossel-Lewis Approach to Chemical Bonding (NCERT)
The electronic configuration of four atoms are given in brackets:
L(1s22s22p1);
M(1s22s22p5);
Q(1s22s22p63s1);
R(1s22s22p2);
The element that would most readily form a diatomic molecule is
M(1s22s22p5);
Q(1s22s22p63s1);
R(1s22s22p2);
Two elements P and Q combine to form a compound. If P has 2 and Q has 6 electrons in their outermost shell, what will be formula of the compound formed?
How many number of electrons are involved in the formation of a nitrogen molecule?
In which of the following molecules octet rule is not followed?
A pair of electrons present between two identicalnon-metals.
During a coordinate bond formation,
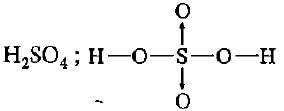
Which of the following molecules contains covalent and coordinate bonds?
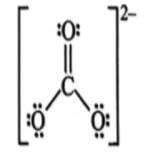
Which of the following shows the Lewis dot formula for CO2?
How many and what types of bonds are present in NH4+?
What is the formal charge on carbon atom in the following two structures?

|
237 docs|243 tests
|