Test: Main Group - 1 - Chemistry MCQ
30 Questions MCQ Test - Test: Main Group - 1
Number of three–centre two–electron (3c–2e) bonds present in diborane is
Which one of the following order of the carbonates is correct for their decomposition temperature:
The characteristics of the blue solution of solution of sodium in liquid ammonia is/are:
Do you expect the minimum energy necessary to eject a 3s electron from phosphorus in a photoelectron spectroscopy experiment for the process.
[Ne] 3s23p3 → [Ne] 3s13p3 + e- ,
Be larger than, smaller than, or the same as the 4th io nization energy (IE4) of phosphorus:
Which of the following will be strongest acid in pure liquid HF:
The melting point of lithium metal is 454 K, and that of sodium is 371 K. Which is the following statements can explain this difference in their melt ing points:
(I) Metallic bonding in lithium is stronger than metallic bonding in sodium.
(II) The delocalized electrons are more strongly attracted to the metal cation of lithium.
(III) The lithium cations have a greater charge density than sodium cation.
(IV) Li+ cations are smaller than Na+ cations.
Although both NF3 and NCl3 are covalent, NCl3 undergoes hydrolysis whereas NF3 does not because:
The Lewis acidity of BF3 is less than BCl3 even though fluorine is more electronegative than chlorine. It is due to:
Pyroenes are a class of silicate minerals, which exhibit a polymeric chain structure, as shown below:
Its simplest repeat unit is
In the following reactions:
The reagent/conditions X and Y are:
Among the following, the group of molecules that undergoes rapid hydrolysis is:
For Et2AlX (X = PPh2–, Ph–, Cl– –and H–), the tendency towards dimeric structure fo llows the order:
Na2HPO4 and NaH2PO4 on heating at high temperature produce a chain sodium pentaphosphate quantitatively.
Q.
The ideal molar ratio of Na2HPO4 to NaH2PO4 is:
Na2HPO4 and NaH2PO4 on heating at high temperature produce a chain sodium pentaphosphate quantitatively.
Q.
The total charge on pentaphosphate anion is:
Among the following substituted silanes, the one that gives cross–linked silicone polymer upon hydrolysis is:
Among the following donors, the one that forms most stable adduct with the Lewis acid B(CH3)3 is:
If a mixture of NaCl, conc. H2SO4 and K2Cr2O7 is heated in a dry test tube, a red vapour (P) is formed. This vapour (P) dissolves in aqueous NaOH to form a yellow solution, which upon treatment with AgNO3 forms a red solid (Q). P and Q are, respectively:
Heating a mixture of ammonium chloride and sodium tetrahydridoborate gives one liquid product(X), along with other products under ambient conditions.
NH4Cl + NaBH4 ------------> X + H2 + NaCl
Q.
Compound X is:
Heating a mixture of ammonium chloride and sodium tetrahydridoborate gives one liquid product(X), along with other products under ambient conditions.
NH4Cl + NaBH4 ------------> X + H2 + NaCl
Q.
Compound X is an example of:
The ease of formation of the adduct, NH3 · BX3 (where X = F, Cl, Br) follows the order:
Aqua regia is a powerful oxidizing agent because it contains:



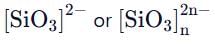
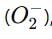 which is an oxygen molecule (O₂) with an extra electron.
which is an oxygen molecule (O₂) with an extra electron.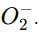 When potassium reacts with oxygen, it forms the superoxide ion in the compound.
When potassium reacts with oxygen, it forms the superoxide ion in the compound.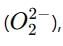 not the superoxide ion.
not the superoxide ion.










