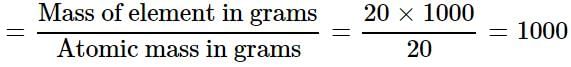Test: Mole Concept & Molar Mass (NCERT) - NEET MCQ
25 Questions MCQ Test - Test: Mole Concept & Molar Mass (NCERT)
What is the mass of carbon dioxide which contains the same number of molecules as are contained in 40 g of oxygen?
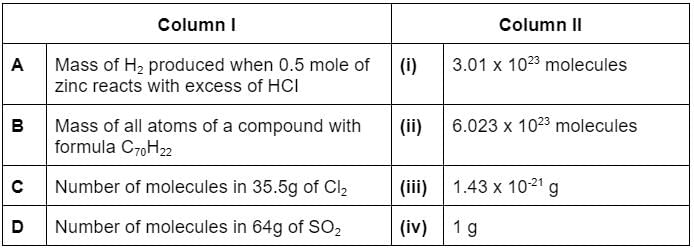
Match the column I with column II and mark the appropriate choice.

The number of oxygen atoms present in 1 mole of oxalic acid dihydrate is
The density of a gas is 1.78 g L-1 at STP. The weight of one mole of gas is
Which of the following gases will have least volume if 10 g of each gas is taken at same temperature and pressure?
How many number of molecules and atoms respectively are present in 2.8 litres of a diatomic gas at STP?
Total number of atoms present in 34g of NH3 is
What will be the mass of 100 atoms of hydrogen?
How many atoms in total are present in 1 kg of sugar?
How many moles of oxygen gas can be produced during electrolytic decomposition of 180 g of water?
1.4 moles of phosphorus trichloride are present in a sample. How many atoms are there in the sample?
What will be the standard molar volume of He, if its density is 0.1784 g/L at STP?
In a mixture of gases, the volume content of a gas is 0.06% at STP. Calculate the number of molecules of the gas in 1 L of the mixture.
What will be the weight of CO having the same number of oxygen atoms as present in 22 g of CO2?
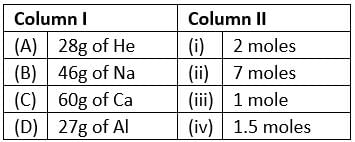
Match the mass of elements given in column I with the no. of moles given in column II and mark the appropriate choice.
How many number of aluminium ions are present in 0.051 g of aluminium oxide?
Which of the following correctly represents 180 g of water?
(i) 5 moles of water
(ii) 10 moles of water
(iii) 6.023 x 1023 molecules ofwater
(iv) 6.023 x 1024 molecules of water
How many grams of CaO are required to react with 852 g of P4O10?
How many oxygen atoms will be present in 88 g of CO2?
A mixture having 2 g of hydrogen and 32 g of oxygen occupies how much volume at NTP?
One atom of an element weighs 3.32 x 10-23 g. How many number of gram atoms are there in 20 kg of the element?
Which of the following statements about Avogadro's hypothesis is correct?
Fill in the blanks by choosing the correct options.
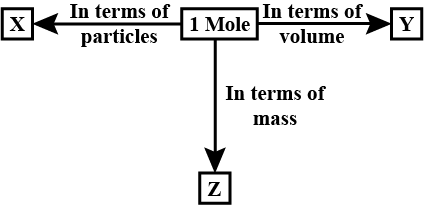
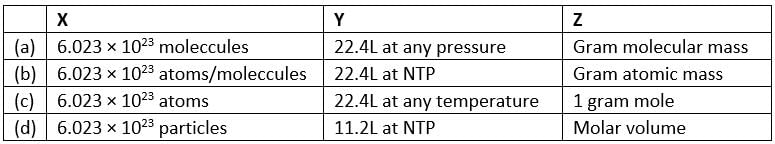
The mass of one mole of a substance in grams is called its
What is the total number of electrons present in 1.6 g of methane?


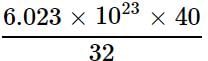 = 7.529 x 1023 molecules
= 7.529 x 1023 molecules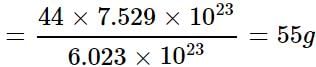

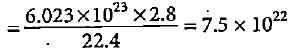 molecules
molecules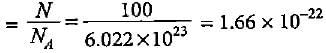
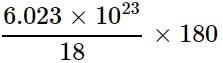 = 6.023 x 1024 molecules of H2O
= 6.023 x 1024 molecules of H2O