Test: Organometallic Chemistry- 2 - Chemistry MCQ
30 Questions MCQ Test - Test: Organometallic Chemistry- 2
Which one of the following has an optical isomer:
The existence of two different coloured complexes with the composition of [Co(NH3)4Cl2]+ is due to:
Which one of the following complexes is not expected to exhibit isomerism:
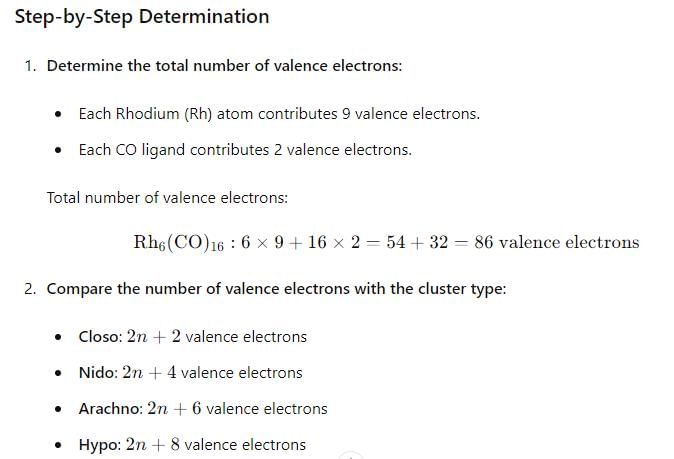
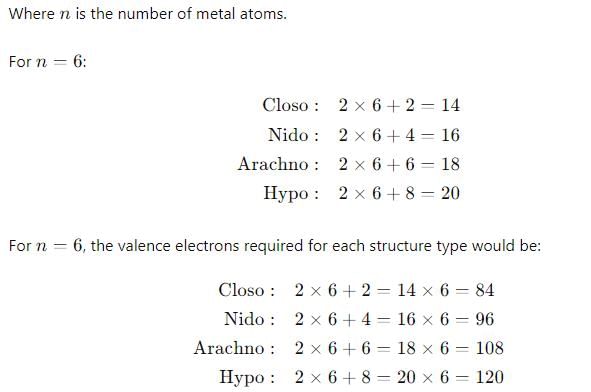
The number of metal-metal bonds in [W2(OPh)6] is:
Among the following which option is correct about the thermal stability of given compounds:
(P) HMn(CO)5
(Q) HTe(CO)5
(R) HRe(CO)5
Which of the following is correct about the basicity:
(P) [Mn(CO)5]–
(Q) [Tc(CO)5]–
(R) [Re(CO)5]–
The number of IR bands will be obtain in M(CO)5
Nitrosyl ligand binds to d-metal atoms in linear and bent fashion and behaves, respectively, as
The correct order of νNO (cm–1) in the following compounds is:
If the bond length of CO bond in carbon monoxide is 1.128 Å. Then what is the value of CO bond length in Fe(CO)5:
Which of the following in not suitable as catalyst for hydroformylation:
The catalyst used for the oxidation of ethylene to acetaldehyde is:
Regarding the catalytic cycle of hydrogenation of alkanes involving RhCl(PPh3)3 as t he catalyst, the correct statement is:
The catalyst used in the conversion of ethylene to acetaldehyde using Wacker process:
The homogeneous catalyst that is used in the hydroformylation or hydrocarbonylation is based on:
Metals used in automobile catalytic converters are:
Active catalytic species for hydroformylation is:
An intermediate formed during the hydroformylation of olefins using Co2(CO)8 as catalyst is:
In Monsanto acetic acid process shown below, the role of HI is:
The reaction of acetyl chloride and AlCl3 with ferrocene gives:
The infra–red starching frequency vCO of P-S follows the order:
Ferrocene undergoes Vilsmeir reaction to yield:
In hydroformylation process, propene is converted into
Which second row transition metal is present in the following compound:
The rate of alkene coordination to [PtCl4]2- is highest for





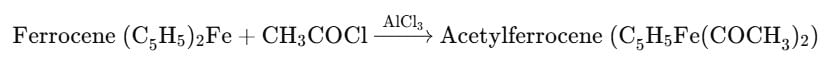
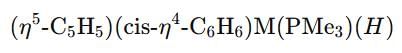
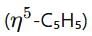 and cyclohexadienyl
and cyclohexadienyl 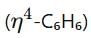 ligands, along with phosphine (PMe3) and hydride (H) groups. The central metal is likely a second-row transition metal from the periodic table, and the coordination environment suggests it is from group 6.
ligands, along with phosphine (PMe3) and hydride (H) groups. The central metal is likely a second-row transition metal from the periodic table, and the coordination environment suggests it is from group 6.















