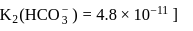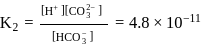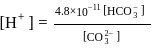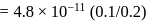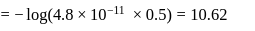Test: PH of Solutions - JEE MCQ
15 Questions MCQ Test - Test: PH of Solutions
The pH of a 0.1 molar solution of the acid HQ is 3. The value of the ionization constant, Ka of this acid is -
[AIEEE-2012]
How many litres of water must be added to litre of an aqueous solution of HCl with a pH of 1 to create an aqueous solution with pH of 2?
[AIEEE-2013]
If the 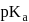 of a weak acid
of a weak acid  is
is 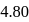 and the
and the 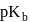 of a weak base
of a weak base 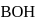 is
is  Then, the
Then, the  of an aqueous solution of the corresponding salt,
of an aqueous solution of the corresponding salt, 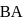 will be
will be
The equlibrium constant of the reaction of a weak acid (HA) with a strong base 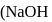 ) is
) is 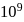 at
at 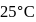 . Hence
. Hence 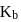 for
for 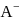 will be equal to
will be equal to
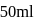 of
of 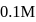 HA is titrated with
HA is titrated with 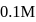 strong base (NaOH). The minimum value of equilibriwn constant (k) so that when
strong base (NaOH). The minimum value of equilibriwn constant (k) so that when 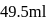 of titrant has been added, the reaction between
of titrant has been added, the reaction between 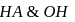 is essentially complete and the
is essentially complete and the 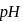 changes by
changes by 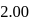 units on addition of two more drops
units on addition of two more drops 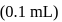 of titrant is
of titrant is
Calculate the ratio of 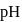 of a solution containing
of a solution containing  of
of 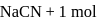 of
of 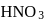 per liter and of other solution containing lmol NaCN + lmol of HCN per liter
per liter and of other solution containing lmol NaCN + lmol of HCN per liter
Given 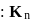 of
of 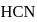 is the order of 10-10.
is the order of 10-10.
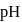 of
of 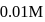 solution of acetic acid is 5.0. What are the values of
solution of acetic acid is 5.0. What are the values of 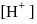 and
and 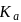 respectively?
respectively?
What volume of 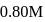 NaOH must be added to
NaOH must be added to 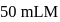 solution of
solution of  , a weak acid
, a weak acid 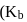 for
for 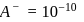 and
and 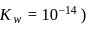 so as to adjust the
so as to adjust the 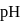 of the mixture at
of the mixture at 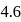 ?
?
If a 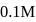 solution of HCN is
solution of HCN is 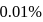 ionised, the ioniation constant for
ionised, the ioniation constant for 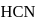 is
is
The 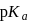 of a weak acid, benzoic acid and
of a weak acid, benzoic acid and 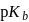 of a weak base, ammonium hydroxide are 4.25 and 4.75 respectively. Then the
of a weak base, ammonium hydroxide are 4.25 and 4.75 respectively. Then the 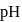 of
of  solution of ammonium benzoate will be
solution of ammonium benzoate will be
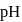 and
and 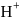 ion concentration is incorrect?
ion concentration is incorrect?



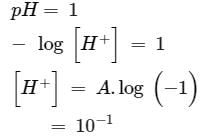
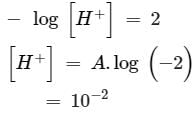
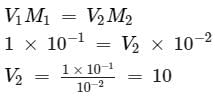

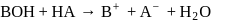
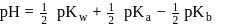
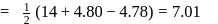
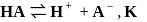
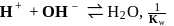
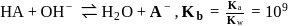
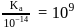 so
so 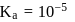
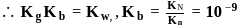
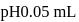 beyond the equivalence point can be calculated as follows
beyond the equivalence point can be calculated as follows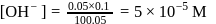
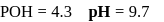 before
before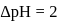 units, the
units, the 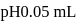 before the equivalence point must be
before the equivalence point must be 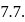 At this point if the
At this point if the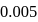 m,mol of HA unreacted,
m,mol of HA unreacted,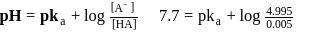
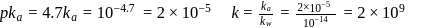
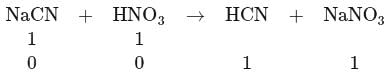
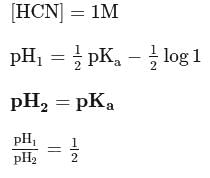
 of
of 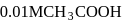 solution
solution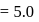
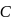 of the solution
of the solution 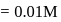
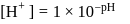
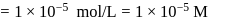
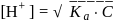
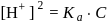
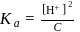
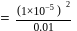
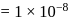
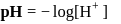
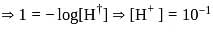 which is higher than
which is higher than 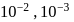 and
and 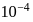
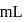 of
of 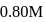 NaOH be added then
NaOH be added then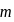 mole of salt
mole of salt 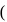 NaA
NaA 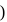 formed
formed 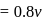
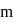 mole of acid remaining unreacted
mole of acid remaining unreacted 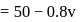
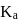 for
for 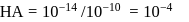
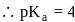
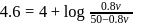
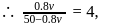 So
So 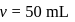
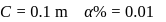
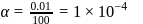
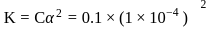
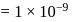
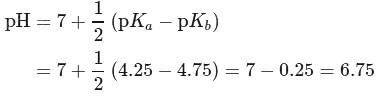
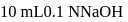 and
and 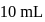
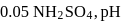 of this solution is :
of this solution is :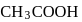 is weak acid while
is weak acid while 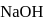 is strong base, so one equivalent of
is strong base, so one equivalent of 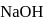 can not be neutralized with one equivalent of
can not be neutralized with one equivalent of 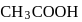 . Hence the solution of one equivalent of each does not have
. Hence the solution of one equivalent of each does not have 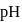 value as 7 . Its
value as 7 . Its 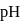 will be towards basic side as
will be towards basic side as 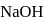 is a strong base hence conc. of
is a strong base hence conc. of  will be more than the conc. of
will be more than the conc. of 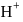
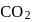 and
and 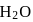 is
is 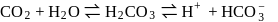
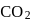 escapes from the system
escapes from the system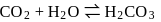
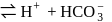
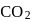 escapes, the equilibrium will shift to LHS and
escapes, the equilibrium will shift to LHS and 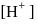 concentration will decrease
concentration will decrease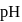 of a solution containing
of a solution containing 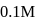
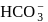 and
and 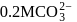
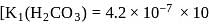 and
and