NEET Exam > NEET Tests > Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - NEET MCQ
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - NEET MCQ
Test Description
10 Questions MCQ Test - Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28)
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) for NEET 2025 is part of NEET preparation. The Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) questions and answers have been prepared
according to the NEET exam syllabus.The Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) MCQs are made for NEET 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) below.
Solutions of Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) questions in English are available as part of our course for NEET & Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) | 10 questions in 20 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 1
What is the oxidation half reaction of Cu+2 + Zn → Cu + Zn+2?
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 1
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 2
When a zinc rod is kept in a copper nitrate solution what happens?
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 2
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 3
In this reaction Cu+2 + Zn → Cu + Zn+2, what is an oxidising agent?
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 3
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 4
Loss of electrons is _________________
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 4
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 5
Which of the following is true as per the metal activity series?
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 5
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 6
Intensity of blue color increases gradually when _________________
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 6
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 7
What is a reducing agent of the reaction Cu+2 + Zn → Cu + Zn+2?
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 7
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 8
Which of the following is not an oxidising agent?
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 8
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 9
Hydrogen peroxide is a ______________
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 9
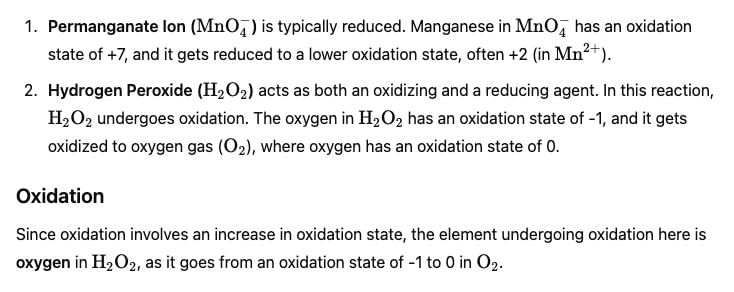
Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 10
In the reaction between potassium permanganate (KMnO4) and hydrogen peroxide (H2O2) in an acidic medium, which element undergoes oxidation?
Detailed Solution for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) - Question 10
Information about Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) Page
In this test you can find the Exam questions for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Redox Reactions in Terms of Electron Transfer Reactions (August 28), EduRev gives you an ample number of Online tests for practice
Download as PDF