Test: Reformatsky, Witting Benzil-Benzilic Acid Rearrangements - JEE MCQ
20 Questions MCQ Test - Test: Reformatsky, Witting Benzil-Benzilic Acid Rearrangements
Only One Option Correct Type
Direction (Q, Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
What is the major product of the following reaction?
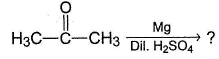
Predict the major product of the following reaction
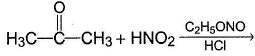
What is formed as predominant product in the following reaction?
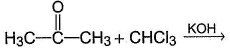
Consider the following sequence of reaction,
Q.
If X is the major organic product, it must be
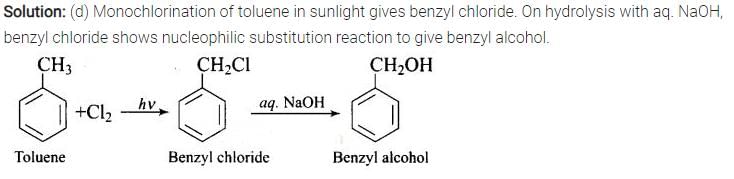
Monochlorination of toluene in sunlight followed by hydrolysis with aq. NaOH yields.
In the given reaction,
Q.
If X is a condensation product then it must be
Consider the following reaction,
In the reaction given below, the final product X is
Direction (Q. Nos. 9-14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE am correct.
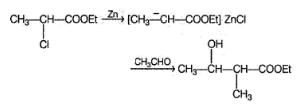
Consider the following Reformatsky reaction
Q.
The correct statements concerning the above reaction is/are
Consider the following chain reaction
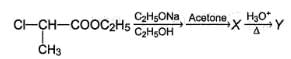
Q.
The correct statements concerning the above reaction is/are
Consider the following sequence of reaction,
Q.
The correct statement(s) regarding the above reaction is/are
Consider the reaction sequence given below,
Q.
The correct statements regarding the above reaction is/are
Consider the reaction sequence given below,
Q.
The correct deductions is/are
Consider the following trans formation,
Comprehension Type
Direction (Q. Nos. 15-17) This sections contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Benzaldehyde when treated with KCN(aq) undergoes a condensation reaction, known as benzoin condensation forming benzoin A. Benzoin on oxidation with HNO3 gives a diketone known as benzil B. Benzil on heating with ethanolic KOH undergo benzil-benzilic acid rearrangement as
The widely accepted mechanism of last step rearrangement is
Q.
When benzil is treated with dilute both of its oxygens are replaced by O18 isotope. It proves that
Benzaldehyde when treated with KCN(aq) undergoes a condensation reaction, known as benzoin condensation forming benzoin A. Benzoin on oxidation with HNO3 gives a diketone known as benzil B. Benzil on heating with ethanolic KOH undergo benzil-benzilic acid rearrangement as
The widely accepted mechanism of last step rearrangement is
Q.
In the following reaction (*C is C14 isotope)
The expected organic products is/are
Benzaldehyde when treated with KCN(aq) undergoes a condensation reaction, known as benzoin condensation forming benzoin A. Benzoin on oxidation with HNO3 gives a diketone known as benzil B. Benzil on heating with ethanolic KOH undergo benzil-benzilic acid rearrangement as
The widely accepted mechanism of last step rearrangement is
Q.
In the reaction given below,
The major rearrangement product is
One Integer Value Correct Type
Direction (Q. Nos. 18-20) This section contains 3 questions. When worked out will result in an integer from 0 fo 9 (both inclusive).
Consider the following two step synthesis:
Q.
If X is finally treated with excess of NaBH4 followed by acid work-up, how many different isomers of diols would be formed?
Consider the reaction given below,
Q.
In the above reaction, how many different isomers of esters are formed?
Consider the reaction given below,
Q.
How many different isomeric hydrocarbons of Y are formed?