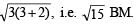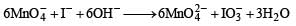Test: Single Correct MCQs: The d- and f-Block Elements & Coordination Compounds | JEE Advanced - JEE MCQ
30 Questions MCQ Test - Test: Single Correct MCQs: The d- and f-Block Elements & Coordination Compounds | JEE Advanced
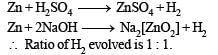
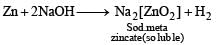
When same amount of zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide, the ratio of volume of hydrogen evolved is
Which of the following is the weakest base
One of the constituent of German silver is
Which of the following dissolve in hot conc. NaOH solution
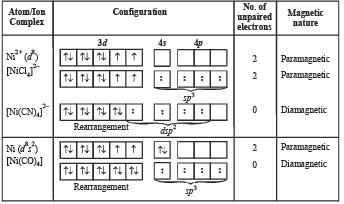
How many unpaired electrons are present in Ni2+?
Sodium thiosulphate is used in photography because of its
Iron is rendered passive by treatment with concentrated
In the metallurgy of iron, when limestone is added to the blast furnace, the calcium ion ends up in
Zinc-copper couple that can be used as a reducing agent is obtained by :
Amongst the following, the lowest degree of paramagnetism per mole of the compound at 298 K will be shown by
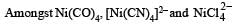
Which one is solder ?
Which pair gives Cl2 at room temperature?
Which compound is formed when excess of KCN is added to aqueous solution of copper sulphate?
Which compound does not dissolve in hot, dilute HNO3?
An aqueous solution of FeSO4, Al2(SO4)3 and chrome alum is heated with excess of Na2O2 and filtered. The materials obtained are :
Ammonium dichromate is used in some fireworks. The green coloured powder blown in the air is
The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is
Which of the following is an organometallic compound?
Which of the following compounds is expected to be coloured?
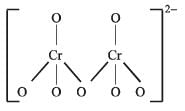
In the dichromate anion,
The geometry of Ni(CO)4 and Ni(PPh3)2Cl2 are
The chemical processes in the production of steel from haematite ore involve
The complex ion which has no ‘d’ electron in the central metal atom is
Anhydrous ferric chloride is prepared by
When MnO2 is fused with KOH, a coloured compound is formed, the product and its colour is:
In the process of extraction of gold,
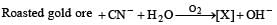
[X ] + Zn → [Y] + Au
Identify the complexes [X] and [Y]
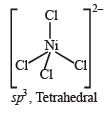
The species having tetrahedral shape is
The spin magnetic moment of cobalt in the compound Hg[Co(SCN)4] is
The product of oxidation of I– with 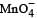 in alkaline medium is
in alkaline medium is



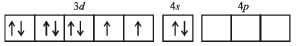
 It has 2 unpaired electrons. 3d orbital of Ni2+ ion. At No. of Ni = 28.
It has 2 unpaired electrons. 3d orbital of Ni2+ ion. At No. of Ni = 28.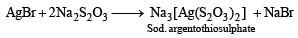
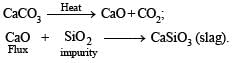
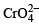 ion and residue is brown due to Fe(OH)3.
ion and residue is brown due to Fe(OH)3.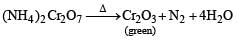
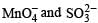 in acidic medium is derived as follows:
in acidic medium is derived as follows: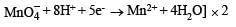
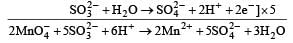
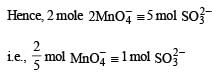
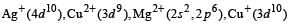 Only Cu2+ ion has one unpaired electron in 3d orbital and so, its salt is expected to be coloured.
Only Cu2+ ion has one unpaired electron in 3d orbital and so, its salt is expected to be coloured.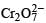
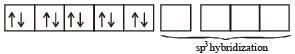
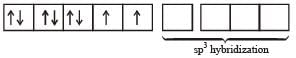
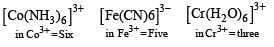
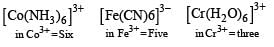
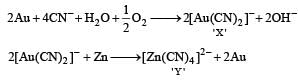
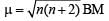 (m= spin magnetic moment) Here Co is present as Co2+ ion which has 3 unpaired electrons. So the spin magnetic moment will be
(m= spin magnetic moment) Here Co is present as Co2+ ion which has 3 unpaired electrons. So the spin magnetic moment will be