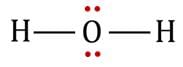Test: VSEPR Theory, Hybridisation (May 24) - Year 11 MCQ
10 Questions MCQ Test - Test: VSEPR Theory, Hybridisation (May 24)
The decreasing order of the repulsive interactions between various electron pairs is:
The s-orbital does not show preference to any direction because
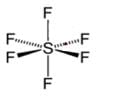
Among the following species octahedral shape is found in:
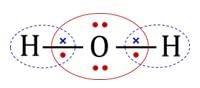
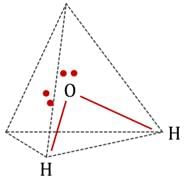
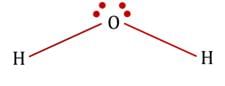
The statement that is true about H2O molecule is:
The species having pyramidal shape is
[IIT JEE 2010]
ECI3 (where, E = B, P, As, Bi) of these elements are known.
Bond angles are in the following order
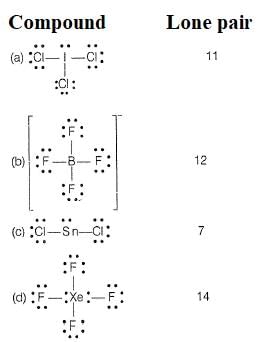
Which of the following molecule/species has the minimum number of lone pairs?
Which of the following set of molecules have the same shape but different hybridisation?
Direction (Q. Nos. 21-22) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF-6)
Q.
Select the correct statements.