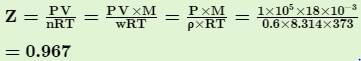Test: Van der Waals Equation (Old NCERT) - JEE MCQ
21 Questions MCQ Test - Test: Van der Waals Equation (Old NCERT)
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. In van der Waals’ equation of state for non-ideal gas, that includes correction for intermolecular forces is
[ITT JEE 1998, 2009]
The value of van der Waals’ constant a for various gases are given
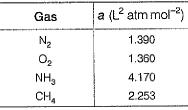
Q. The order of liquefaction of these gases is
[IITJEE 1989]
A gas will approach ideal behavious at
[IIT JEE 1999]
The compressibility of a gas is less than unity for a gas at STP. Therefore,
[IIT JEE 2000]
Positive deviation from ideal behaviour takes place because of
[IIT JEE 2003]
Van der Waals ’ equation for one mole of CO2 gas at low pressure will be
[JEE Advanced 2014]
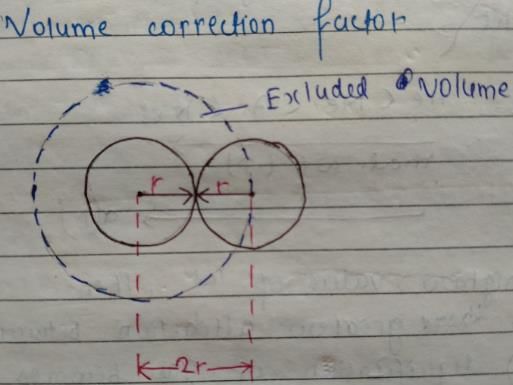
If V is the volume of one molecule of a gas under given conditions, then van der Waals ’ constant b (also called excluded volume or effective volume) is
Fraction of the effective volume occupied by molecules in 1 mole of a gas at STP (diameter of the molecule is 2 x 10-10m) is
The compressibility factors for real gases at low pressure , high pressure and that of gases of low molar masses are Z1 Z2 and Z3. These are
[AIEEE 2012 , JEE Main 2014]
a and b are van der Waals’ constants for gases. Chlorine is more easily liquefied than ethane because
[AIEEE 2011]
For one mole of a van der Waals’ gas when b = 0 and T = 300 K, the pV versus 1/V plot is shown in figure. The value of the van der Waals' constant a (atm L2 mol-2) is
Direction (Q. Nos. 12) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q. Match the parameters given in Column I with the units given in Column II and select the correct answer from the codes given below.
Direction (Q. Nos. 13-15) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. The given graph represent the variation of Z versus p, for three real gases A, B and C. Select correct statement(s).
When two gaseous molecules of equal radius r collide,
Select the correct statements based on the virial equation .
The coefficient B in the equation of state
Direction (Q. Nos. 16 and 17) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : At constant temperature, pV versus p isotherm for real gases is not a straight line.
Statement II : At high pressure, all gases have Z > 1, but at intermediate pressure, most gases have Z < 1.
Statement I : In van der Waals’ equation, pressure correction is due to force of attraction between molecules.
Statement II : Rate of change of momentum is equal to force.
Direction (Q. Nos. 18-20) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
For CO, variation of pV with p at constant temperature is given by isotherm shown in figure.
Q. Near the point A, compressibility factor Z is
For CO, variation of pV with p at constant temperature is given by isotherm shown in figure.
Q. Near the point B, Compresibility factor Z is
For CO, variation of pV with p at constant temperature is given by isotherm shown in figure.
Q. For which gas. Z has the same relation as at point B
Direction (Q. Nos. 21) This section contains 1 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. The density of steam at 100.0°C and 1.0x 105 Pa is 0.6 kg m-3. Thus, compressibility factor (Z) deviates from the normal value under ideal condition is ...... %.



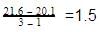
 The reason for this is that for real gas, we have to consider the intermolecular forces which are ignored in ideal gas.
The reason for this is that for real gas, we have to consider the intermolecular forces which are ignored in ideal gas.